Graphynes: how they are hydrogenated
Graphyne is a generic name for a carbon allotrope family of 2D structures, where acetylenic groups connect benzenoid rings, with the coexistence of sp and sp2 hybridized carbon atoms. In this work we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics and structural changes of the hydrogenation of α, β, and γ graphyne forms. Our results showed that the existence of different sites for hydrogen bonding, related to single and triple bonds, makes the process of incorporating hydrogen atoms into graphyne membranes much more complex than the graphene ones. Our results also show that hydrogenation reactions are strongly site dependent and that the sp-hybridized carbon atoms are the preferential sites to chemical attacks. In our cases, the effectiveness of the hydrogenation (estimated from the number of hydrogen atoms covalently bonded to carbon atoms) follows the α, β, γ-graphyne structure ordering.
See more details in published article:
Best wishes.
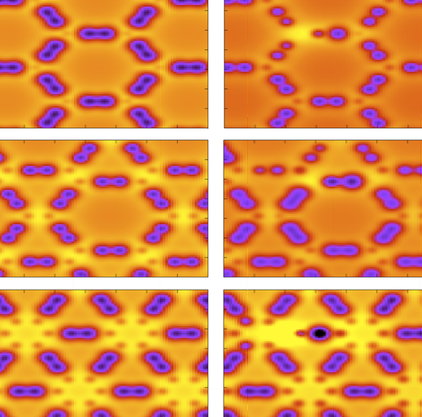 Site Dependent Hydrogenation in Graphynes: A Fully Atomistic Molecular Dynamics Investigation. In: Mater. Res. Soc. Symp. Proc. , vol. 1726 , 2015.
Site Dependent Hydrogenation in Graphynes: A Fully Atomistic Molecular Dynamics Investigation. In: Mater. Res. Soc. Symp. Proc. , vol. 1726 , 2015.



Comentários