1.
Paupitz, R; Autreto, Pedro AS; Legoas, SB; Srinivasan, S Goverapet; van Duin, Adri CT; Galvao, DS
Graphene to fluorographene and fluorographane: a theoretical study Journal Article
Em: Nanotechnology, vol. 24, não 3, pp. 035706, 2013.
@article{Paupitz2013,
title = {Graphene to fluorographene and fluorographane: a theoretical study},
author = {Paupitz, R and Autreto, Pedro AS and Legoas, SB and Srinivasan, S Goverapet and van Duin, Adri CT and Galvao, DS},
url = {http://iopscience.iop.org/0957-4484/24/3/035706/article?fromSearchPage=true},
year = {2013},
date = {2013-01-01},
journal = {Nanotechnology},
volume = {24},
number = {3},
pages = {035706},
publisher = {IOP Publishing},
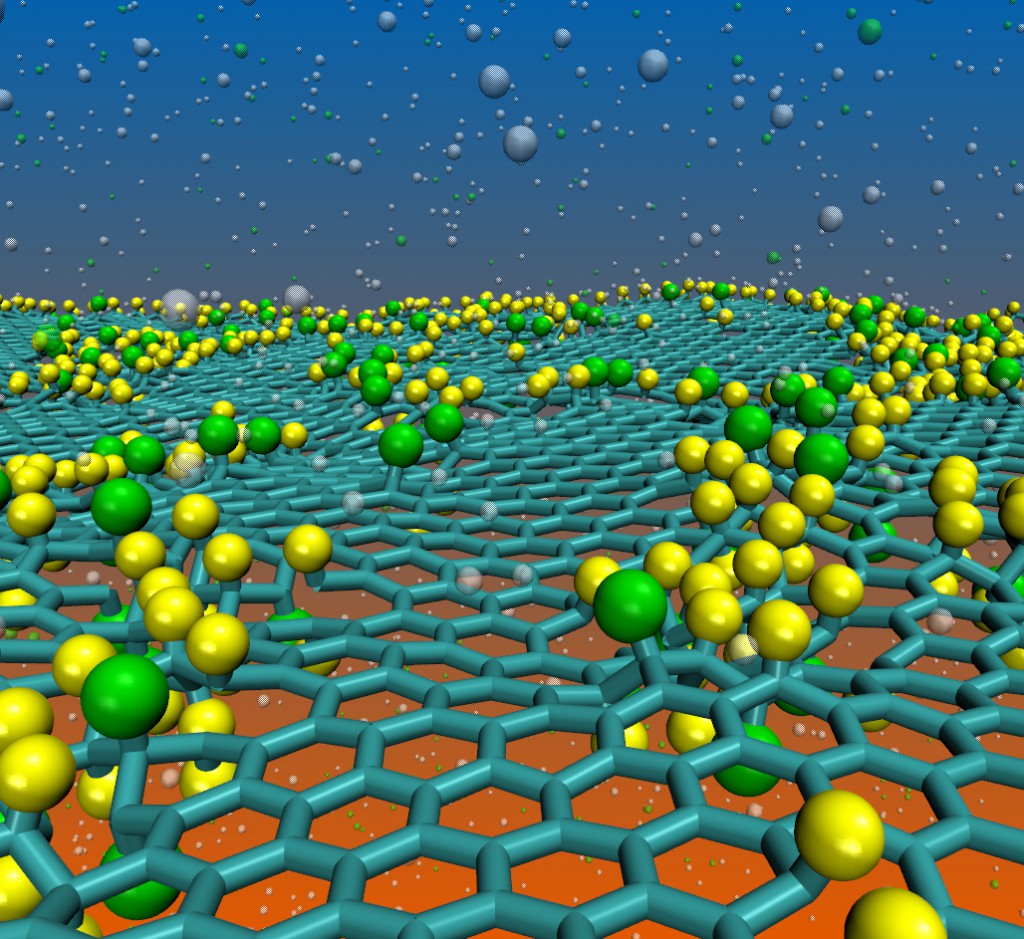
abstract = {We report here a fully reactive molecular dynamics study on the structural and dynamical aspects of the fluorination of graphene membranes (fluorographene). Our results show that fluorination tends to produce defective areas on the graphene membranes with significant distortions of carbon–carbon bonds. Depending on the amount of incorporated fluorine atoms, large membrane holes were observed due to carbon atom losses. These results may explain the broad distribution of the structural lattice parameter values experimentally observed. We have also investigated the effects of mixing hydrogen and fluorine atoms on the graphene functionalization. Our results show that, when in small amounts, the presence of hydrogen atoms produces a significant decrease in the rate of fluorine incorporation onto the membrane. On the other hand, when fluorine is the minority element, it produces a significant catalytic effect on the rate of hydrogen incorporation. We have also observed the spontaneous formation of new hybrid structures with different stable configurations (chair-like, zigzag-like and boat-like) which we named fluorographane.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
We report here a fully reactive molecular dynamics study on the structural and dynamical aspects of the fluorination of graphene membranes (fluorographene). Our results show that fluorination tends to produce defective areas on the graphene membranes with significant distortions of carbon–carbon bonds. Depending on the amount of incorporated fluorine atoms, large membrane holes were observed due to carbon atom losses. These results may explain the broad distribution of the structural lattice parameter values experimentally observed. We have also investigated the effects of mixing hydrogen and fluorine atoms on the graphene functionalization. Our results show that, when in small amounts, the presence of hydrogen atoms produces a significant decrease in the rate of fluorine incorporation onto the membrane. On the other hand, when fluorine is the minority element, it produces a significant catalytic effect on the rate of hydrogen incorporation. We have also observed the spontaneous formation of new hybrid structures with different stable configurations (chair-like, zigzag-like and boat-like) which we named fluorographane.
2013
1.
Paupitz, R; Autreto, Pedro AS; Legoas, SB; Srinivasan, S Goverapet; van Duin, Adri CT; Galvao, DS
Graphene to fluorographene and fluorographane: a theoretical study Journal Article
Em: Nanotechnology, vol. 24, não 3, pp. 035706, 2013.
Resumo | Links | BibTeX | Tags: fluorographane, fluorographene, graphene, molecular dynamics, reaxFF
@article{Paupitz2013,
title = {Graphene to fluorographene and fluorographane: a theoretical study},
author = {Paupitz, R and Autreto, Pedro AS and Legoas, SB and Srinivasan, S Goverapet and van Duin, Adri CT and Galvao, DS},
url = {http://iopscience.iop.org/0957-4484/24/3/035706/article?fromSearchPage=true},
year = {2013},
date = {2013-01-01},
journal = {Nanotechnology},
volume = {24},
number = {3},
pages = {035706},
publisher = {IOP Publishing},
abstract = {We report here a fully reactive molecular dynamics study on the structural and dynamical aspects of the fluorination of graphene membranes (fluorographene). Our results show that fluorination tends to produce defective areas on the graphene membranes with significant distortions of carbon–carbon bonds. Depending on the amount of incorporated fluorine atoms, large membrane holes were observed due to carbon atom losses. These results may explain the broad distribution of the structural lattice parameter values experimentally observed. We have also investigated the effects of mixing hydrogen and fluorine atoms on the graphene functionalization. Our results show that, when in small amounts, the presence of hydrogen atoms produces a significant decrease in the rate of fluorine incorporation onto the membrane. On the other hand, when fluorine is the minority element, it produces a significant catalytic effect on the rate of hydrogen incorporation. We have also observed the spontaneous formation of new hybrid structures with different stable configurations (chair-like, zigzag-like and boat-like) which we named fluorographane.},
keywords = {fluorographane, fluorographene, graphene, molecular dynamics, reaxFF},
pubstate = {published},
tppubtype = {article}
}
We report here a fully reactive molecular dynamics study on the structural and dynamical aspects of the fluorination of graphene membranes (fluorographene). Our results show that fluorination tends to produce defective areas on the graphene membranes with significant distortions of carbon–carbon bonds. Depending on the amount of incorporated fluorine atoms, large membrane holes were observed due to carbon atom losses. These results may explain the broad distribution of the structural lattice parameter values experimentally observed. We have also investigated the effects of mixing hydrogen and fluorine atoms on the graphene functionalization. Our results show that, when in small amounts, the presence of hydrogen atoms produces a significant decrease in the rate of fluorine incorporation onto the membrane. On the other hand, when fluorine is the minority element, it produces a significant catalytic effect on the rate of hydrogen incorporation. We have also observed the spontaneous formation of new hybrid structures with different stable configurations (chair-like, zigzag-like and boat-like) which we named fluorographane.


