1.
Braga, SF; Galvao, DS
A semiempirical study on the electronic structure of 10-deacetylbaccatin-III Journal Article
In: Journal of Molecular Graphics and Modelling, vol. 21, no. 1, pp. 57–70, 2002.
@article{braga2002semiempirical,
title = {A semiempirical study on the electronic structure of 10-deacetylbaccatin-III},
author = {Braga, SF and Galvao, DS},
url = {http://www.sciencedirect.com/science/article/pii/S1093326302001213},
year = {2002},
date = {2002-01-01},
journal = {Journal of Molecular Graphics and Modelling},
volume = {21},
number = {1},
pages = {57--70},
publisher = {Elsevier},
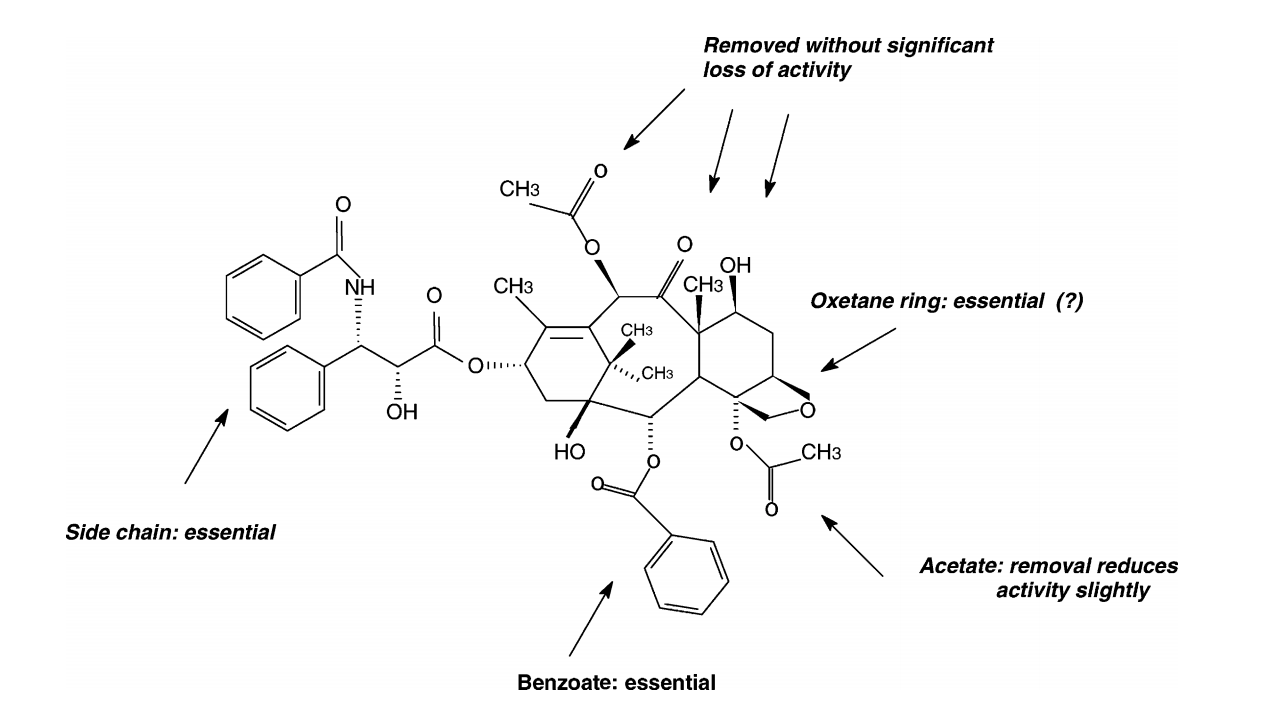
abstract = {We performed a conformational and electronic analysis for 10-deacetylbaccatin-III (DBAC) using well-known semiempirical methods (parametric method 3 (PM3) and Zerner’s intermediate neglect of differential overlap (ZINDO)) coupled to the concepts of total and local density of states (LDOS). Our results indicate that regions presented by paclitaxel (Taxol®) as important for the biological activity can be traced out by the electronic features present in DBAC. These molecules differ only by a phenylisoserine side chain. Compared to paclitaxel, DBAC has a simpler structure in terms of molecular size and number of degrees of freedom (d.f.). This makes DBAC a good candidate for a preliminary investigation of the taxoid family. Our results question the importance of the oxetane group, which seems to be consistent with recent experimental data.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
We performed a conformational and electronic analysis for 10-deacetylbaccatin-III (DBAC) using well-known semiempirical methods (parametric method 3 (PM3) and Zerner’s intermediate neglect of differential overlap (ZINDO)) coupled to the concepts of total and local density of states (LDOS). Our results indicate that regions presented by paclitaxel (Taxol®) as important for the biological activity can be traced out by the electronic features present in DBAC. These molecules differ only by a phenylisoserine side chain. Compared to paclitaxel, DBAC has a simpler structure in terms of molecular size and number of degrees of freedom (d.f.). This makes DBAC a good candidate for a preliminary investigation of the taxoid family. Our results question the importance of the oxetane group, which seems to be consistent with recent experimental data.
2002
1.
Braga, SF; Galvao, DS
A semiempirical study on the electronic structure of 10-deacetylbaccatin-III Journal Article
In: Journal of Molecular Graphics and Modelling, vol. 21, no. 1, pp. 57–70, 2002.
Abstract | Links | BibTeX | Tags: Baccatin, Drug Design, Electronic Structure, Taxol, Taxotere, Theory of Electronic Indices
@article{braga2002semiempirical,
title = {A semiempirical study on the electronic structure of 10-deacetylbaccatin-III},
author = {Braga, SF and Galvao, DS},
url = {http://www.sciencedirect.com/science/article/pii/S1093326302001213},
year = {2002},
date = {2002-01-01},
journal = {Journal of Molecular Graphics and Modelling},
volume = {21},
number = {1},
pages = {57--70},
publisher = {Elsevier},
abstract = {We performed a conformational and electronic analysis for 10-deacetylbaccatin-III (DBAC) using well-known semiempirical methods (parametric method 3 (PM3) and Zerner’s intermediate neglect of differential overlap (ZINDO)) coupled to the concepts of total and local density of states (LDOS). Our results indicate that regions presented by paclitaxel (Taxol®) as important for the biological activity can be traced out by the electronic features present in DBAC. These molecules differ only by a phenylisoserine side chain. Compared to paclitaxel, DBAC has a simpler structure in terms of molecular size and number of degrees of freedom (d.f.). This makes DBAC a good candidate for a preliminary investigation of the taxoid family. Our results question the importance of the oxetane group, which seems to be consistent with recent experimental data.
},
keywords = {Baccatin, Drug Design, Electronic Structure, Taxol, Taxotere, Theory of Electronic Indices},
pubstate = {published},
tppubtype = {article}
}
We performed a conformational and electronic analysis for 10-deacetylbaccatin-III (DBAC) using well-known semiempirical methods (parametric method 3 (PM3) and Zerner’s intermediate neglect of differential overlap (ZINDO)) coupled to the concepts of total and local density of states (LDOS). Our results indicate that regions presented by paclitaxel (Taxol®) as important for the biological activity can be traced out by the electronic features present in DBAC. These molecules differ only by a phenylisoserine side chain. Compared to paclitaxel, DBAC has a simpler structure in terms of molecular size and number of degrees of freedom (d.f.). This makes DBAC a good candidate for a preliminary investigation of the taxoid family. Our results question the importance of the oxetane group, which seems to be consistent with recent experimental data.
http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ


