http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ
1.
Chandra Sekhar Tiwary Mohamad A Kabbani, Pedro AS Autreto
Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes Journal Article
Em: Nature Communications, vol. 6, pp. 7291, 2015.
@article{Kabbani2015,
title = {Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes},
author = {Mohamad A Kabbani, Chandra Sekhar Tiwary, Pedro AS Autreto, Gustavo Brunetto, Anirban Som, KR Krishnadas, Sehmus Ozden, Ken P Hackenberg, Yongi Gong, Douglas S Galvao, Robert Vajtai, Ahmad T Kabbani, Thalappil Pradeep, Pulickel M Ajayan},
url = {http://www.nature.com/ncomms/2015/150615/ncomms8291/full/ncomms8291.html},
doi = {10.1038/ncomms8291},
year = {2015},
date = {2015-06-15},
journal = {Nature Communications},
volume = {6},
pages = {7291},
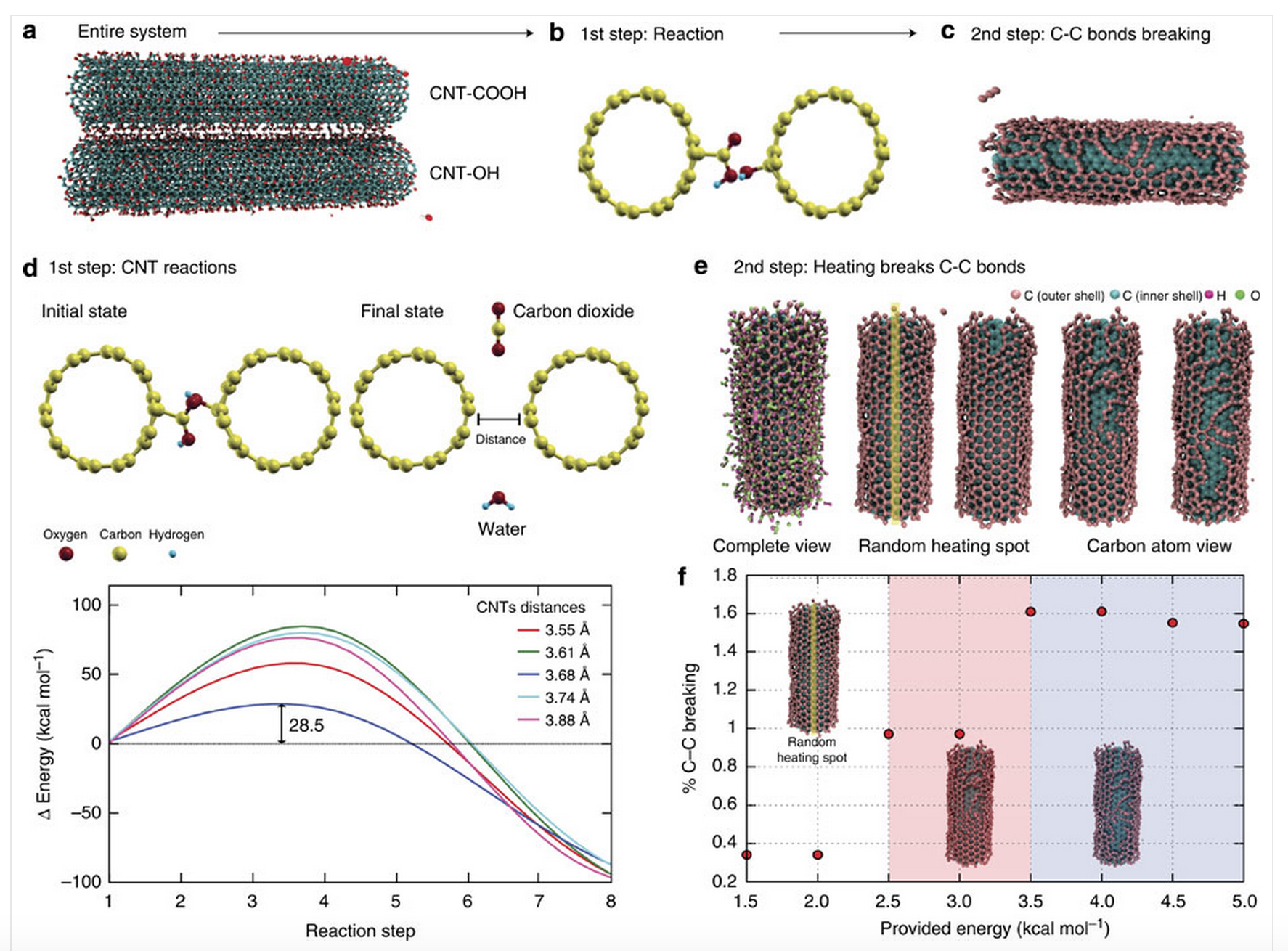
abstract = {Carbon nanotubes can be chemically modified by attaching various functionalities to their surfaces, although harsh chemical treatments can lead to their break-up into graphene nanostructures. On the other hand, direct coupling between functionalities bound on individual nanotubes could lead to, as yet unexplored, spontaneous chemical reactions. Here we report an ambient mechano-chemical reaction between two varieties of nanotubes, carrying predominantly carboxyl and hydroxyl functionalities, respectively, facilitated by simple mechanical grinding of the reactants. The purely solid-state reaction between the chemically differentiated nanotube species produces condensation products and unzipping of nanotubes due to local energy release, as confirmed by spectroscopic measurements, thermal analysis and molecular dynamic simulations.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Carbon nanotubes can be chemically modified by attaching various functionalities to their surfaces, although harsh chemical treatments can lead to their break-up into graphene nanostructures. On the other hand, direct coupling between functionalities bound on individual nanotubes could lead to, as yet unexplored, spontaneous chemical reactions. Here we report an ambient mechano-chemical reaction between two varieties of nanotubes, carrying predominantly carboxyl and hydroxyl functionalities, respectively, facilitated by simple mechanical grinding of the reactants. The purely solid-state reaction between the chemically differentiated nanotube species produces condensation products and unzipping of nanotubes due to local energy release, as confirmed by spectroscopic measurements, thermal analysis and molecular dynamic simulations.
2015
1.
Chandra Sekhar Tiwary Mohamad A Kabbani, Pedro AS Autreto
Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes Journal Article
Em: Nature Communications, vol. 6, pp. 7291, 2015.
Resumo | Links | BibTeX | Tags: Carbon Nanotubes, Chemical Reactions, Electronic Structure, Molecular Dynamics, top20
@article{Kabbani2015,
title = {Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes},
author = {Mohamad A Kabbani, Chandra Sekhar Tiwary, Pedro AS Autreto, Gustavo Brunetto, Anirban Som, KR Krishnadas, Sehmus Ozden, Ken P Hackenberg, Yongi Gong, Douglas S Galvao, Robert Vajtai, Ahmad T Kabbani, Thalappil Pradeep, Pulickel M Ajayan},
url = {http://www.nature.com/ncomms/2015/150615/ncomms8291/full/ncomms8291.html},
doi = {10.1038/ncomms8291},
year = {2015},
date = {2015-06-15},
journal = {Nature Communications},
volume = {6},
pages = {7291},
abstract = {Carbon nanotubes can be chemically modified by attaching various functionalities to their surfaces, although harsh chemical treatments can lead to their break-up into graphene nanostructures. On the other hand, direct coupling between functionalities bound on individual nanotubes could lead to, as yet unexplored, spontaneous chemical reactions. Here we report an ambient mechano-chemical reaction between two varieties of nanotubes, carrying predominantly carboxyl and hydroxyl functionalities, respectively, facilitated by simple mechanical grinding of the reactants. The purely solid-state reaction between the chemically differentiated nanotube species produces condensation products and unzipping of nanotubes due to local energy release, as confirmed by spectroscopic measurements, thermal analysis and molecular dynamic simulations.},
keywords = {Carbon Nanotubes, Chemical Reactions, Electronic Structure, Molecular Dynamics, top20},
pubstate = {published},
tppubtype = {article}
}
Carbon nanotubes can be chemically modified by attaching various functionalities to their surfaces, although harsh chemical treatments can lead to their break-up into graphene nanostructures. On the other hand, direct coupling between functionalities bound on individual nanotubes could lead to, as yet unexplored, spontaneous chemical reactions. Here we report an ambient mechano-chemical reaction between two varieties of nanotubes, carrying predominantly carboxyl and hydroxyl functionalities, respectively, facilitated by simple mechanical grinding of the reactants. The purely solid-state reaction between the chemically differentiated nanotube species produces condensation products and unzipping of nanotubes due to local energy release, as confirmed by spectroscopic measurements, thermal analysis and molecular dynamic simulations.


