http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ
Xifan Wang Sidong Lei, Bo Li
Surface functionalization of two-dimensional metal chalcogenides by Lewis acid–base chemistry Journal Article
Em: Nature Nanotechnology, vol. 11, pp. 465–471, 2016.
@article{Lei2016,
title = {Surface functionalization of two-dimensional metal chalcogenides by Lewis acid–base chemistry},
author = {Sidong Lei, Xifan Wang, Bo Li, Jiahao Kang, Yongmin He, Antony George, Liehui Ge, Yongji Gong, Pei Dong, Zehua Jin, Gustavo Brunetto, Weibing Chen, Zuan-Tao Lin, Robert Baines, Douglas S. Galvão, Jun Lou, Enrique Barrera, Kaustav Banerjee, Robert Vajtai & Pulickel Ajayan},
url = {http://www.nature.com/nnano/journal/vaop/ncurrent/full/nnano.2015.323.html},
doi = {10.1038/nnano.2015.323},
year = {2016},
date = {2016-02-01},
journal = {Nature Nanotechnology},
volume = {11},
pages = {465–471},
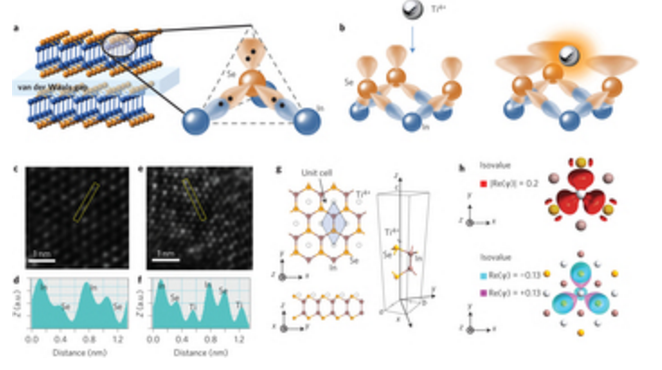
abstract = {Precise control of the electronic surface states of two-dimensional (2D) materials could improve their versatility and widen their applicability in electronics and sensing. To this end, chemical surface functionalization has been used to adjust the electronic properties of 2D materials. So far, however, chemical functionalization has relied on lattice defects and physisorption methods that inevitably modify the topological characteristics of the atomic layers. Here we make use of the lone pair electrons found in most of 2D metal chalcogenides and report a functionalization method via a Lewis acid–base reaction that does not alter the host structure. Atomic layers of n-type InSe react with Ti4+ to form planar p-type [Ti4+n(InSe)] coordination complexes. Using this strategy, we fabricate planar p–n junctions on 2D InSe with improved rectification and photovoltaic properties, without requiring heterostructure growth procedures or device fabrication processes. We also show that this functionalization approach works with other Lewis acids (such as B3+, Al3+ and Sn4+) and can be applied to other 2D materials (for example MoS2, MoSe2). Finally, we show that it is possible to use Lewis acid–base chemistry as a bridge to connect molecules to 2D atomic layers and fabricate a proof-of-principle dye-sensitized photosensing device.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Dong, Pei; Chipara, Alin Cristian; Loya, Phillip; Yang, Yingchao; Ge, Liehui; Lei, Sidong; Li, Bo; Brunetto, Gustavo; Machado, Leonardo Dantas; Hong, Liang; others,
A Solid-liquid Self-adaptive Polymeric Composite Journal Article
Em: ACS Applied Materials & Interfaces, vol. 8, não 3, pp. 2142–2147, 2016.
@article{Dong2016,
title = {A Solid-liquid Self-adaptive Polymeric Composite},
author = {Dong, Pei and Chipara, Alin Cristian and Loya, Phillip and Yang, Yingchao and Ge, Liehui and Lei, Sidong and Li, Bo and Brunetto, Gustavo and Machado, Leonardo Dantas and Hong, Liang and others},
url = {http://pubs.acs.org/doi/abs/10.1021/acsami.5b10667},
doi = {10.1021/acsami.5b10667},
year = {2016},
date = {2016-01-01},
journal = {ACS Applied Materials & Interfaces},
volume = {8},
number = {3},
pages = {2142–2147},
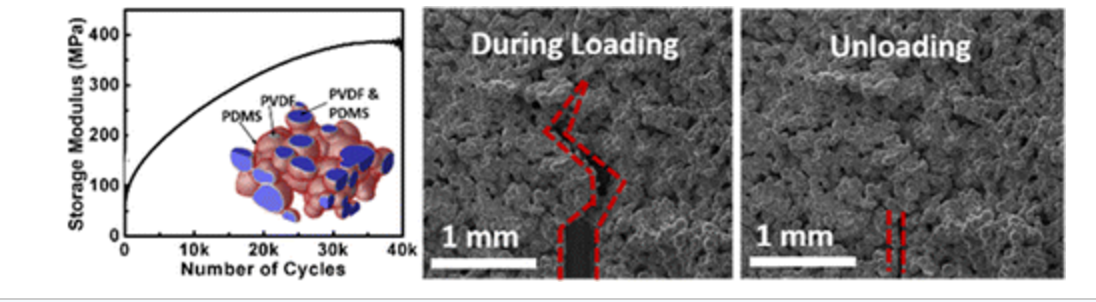
abstract = {A solid–liquid self-adaptive composite (SAC) is synthesized using a simple mixing–evaporation protocol, with poly(dimethylsiloxane) (PDMS) and poly(vinylidene fluoride) (PVDF) as active constituents. SAC exists as a porous solid containing a near equivalent distribution of the solid (PVDF)–liquid (PDMS) phases, with the liquid encapsulated and stabilized within a continuous solid network percolating throughout the structure. The pores, liquid, and solid phases form a complex hierarchical structure, which offers both mechanical robustness and a significant structural adaptability under external forces. SAC exhibits attractive self-healing properties during tension, and demonstrates reversible self-stiffening properties under compression with a maximum of 7-fold increase seen in the storage modulus. In a comparison to existing self-healing and self-stiffening materials, SAC offers distinct advantages in the ease of fabrication, high achievable storage modulus, and reversibility. Such materials could provide a new class of adaptive materials system with multifunctionality, tunability, and scale-up potentials.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016
Xifan Wang Sidong Lei, Bo Li
Surface functionalization of two-dimensional metal chalcogenides by Lewis acid–base chemistry Journal Article
Em: Nature Nanotechnology, vol. 11, pp. 465–471, 2016.
Resumo | Links | BibTeX | Tags: Chalcogenides, Modelling, Synthesis, top20
@article{Lei2016,
title = {Surface functionalization of two-dimensional metal chalcogenides by Lewis acid–base chemistry},
author = {Sidong Lei, Xifan Wang, Bo Li, Jiahao Kang, Yongmin He, Antony George, Liehui Ge, Yongji Gong, Pei Dong, Zehua Jin, Gustavo Brunetto, Weibing Chen, Zuan-Tao Lin, Robert Baines, Douglas S. Galvão, Jun Lou, Enrique Barrera, Kaustav Banerjee, Robert Vajtai & Pulickel Ajayan},
url = {http://www.nature.com/nnano/journal/vaop/ncurrent/full/nnano.2015.323.html},
doi = {10.1038/nnano.2015.323},
year = {2016},
date = {2016-02-01},
journal = {Nature Nanotechnology},
volume = {11},
pages = {465–471},
abstract = {Precise control of the electronic surface states of two-dimensional (2D) materials could improve their versatility and widen their applicability in electronics and sensing. To this end, chemical surface functionalization has been used to adjust the electronic properties of 2D materials. So far, however, chemical functionalization has relied on lattice defects and physisorption methods that inevitably modify the topological characteristics of the atomic layers. Here we make use of the lone pair electrons found in most of 2D metal chalcogenides and report a functionalization method via a Lewis acid–base reaction that does not alter the host structure. Atomic layers of n-type InSe react with Ti4+ to form planar p-type [Ti4+n(InSe)] coordination complexes. Using this strategy, we fabricate planar p–n junctions on 2D InSe with improved rectification and photovoltaic properties, without requiring heterostructure growth procedures or device fabrication processes. We also show that this functionalization approach works with other Lewis acids (such as B3+, Al3+ and Sn4+) and can be applied to other 2D materials (for example MoS2, MoSe2). Finally, we show that it is possible to use Lewis acid–base chemistry as a bridge to connect molecules to 2D atomic layers and fabricate a proof-of-principle dye-sensitized photosensing device.
},
keywords = {Chalcogenides, Modelling, Synthesis, top20},
pubstate = {published},
tppubtype = {article}
}
Dong, Pei; Chipara, Alin Cristian; Loya, Phillip; Yang, Yingchao; Ge, Liehui; Lei, Sidong; Li, Bo; Brunetto, Gustavo; Machado, Leonardo Dantas; Hong, Liang; others,
A Solid-liquid Self-adaptive Polymeric Composite Journal Article
Em: ACS Applied Materials & Interfaces, vol. 8, não 3, pp. 2142–2147, 2016.
Resumo | Links | BibTeX | Tags: Adhesives, Modelling, Polymers
@article{Dong2016,
title = {A Solid-liquid Self-adaptive Polymeric Composite},
author = {Dong, Pei and Chipara, Alin Cristian and Loya, Phillip and Yang, Yingchao and Ge, Liehui and Lei, Sidong and Li, Bo and Brunetto, Gustavo and Machado, Leonardo Dantas and Hong, Liang and others},
url = {http://pubs.acs.org/doi/abs/10.1021/acsami.5b10667},
doi = {10.1021/acsami.5b10667},
year = {2016},
date = {2016-01-01},
journal = {ACS Applied Materials & Interfaces},
volume = {8},
number = {3},
pages = {2142–2147},
abstract = {A solid–liquid self-adaptive composite (SAC) is synthesized using a simple mixing–evaporation protocol, with poly(dimethylsiloxane) (PDMS) and poly(vinylidene fluoride) (PVDF) as active constituents. SAC exists as a porous solid containing a near equivalent distribution of the solid (PVDF)–liquid (PDMS) phases, with the liquid encapsulated and stabilized within a continuous solid network percolating throughout the structure. The pores, liquid, and solid phases form a complex hierarchical structure, which offers both mechanical robustness and a significant structural adaptability under external forces. SAC exhibits attractive self-healing properties during tension, and demonstrates reversible self-stiffening properties under compression with a maximum of 7-fold increase seen in the storage modulus. In a comparison to existing self-healing and self-stiffening materials, SAC offers distinct advantages in the ease of fabrication, high achievable storage modulus, and reversibility. Such materials could provide a new class of adaptive materials system with multifunctionality, tunability, and scale-up potentials.
},
keywords = {Adhesives, Modelling, Polymers},
pubstate = {published},
tppubtype = {article}
}


