http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ
BORGES, Daiane DAMASCENO; NORMAND, Perine; PERMIAKOVA, Anastasia; BABARAO, Ravichandar; HEYMANS, Nicolas; GALVAO, Douglas S.; SERRE, Christian; WEIRELD, Guy DE; MAURIN, Guillaume
Gas Adsorption and Separation by the Al-based Metal-Organic Framework MIL-160 Journal Article
Em: Journal of Physical Chemistry C, vol. 121, não 48, pp. 26822–26832, 2017.
@article{BORGES2017b,
title = {Gas Adsorption and Separation by the Al-based Metal-Organic Framework MIL-160},
author = {Daiane DAMASCENO BORGES and Perine NORMAND and Anastasia PERMIAKOVA and Ravichandar BABARAO and Nicolas HEYMANS and Douglas S. GALVAO and Christian SERRE and Guy DE WEIRELD and Guillaume MAURIN},
url = {http://pubs.acs.org/doi/abs/10.1021/acs.jpcc.7b08856},
doi = {DOI: 10.1021/acs.jpcc.7b08856},
year = {2017},
date = {2017-09-14},
journal = {Journal of Physical Chemistry C},
volume = {121},
number = {48},
pages = {26822–26832},
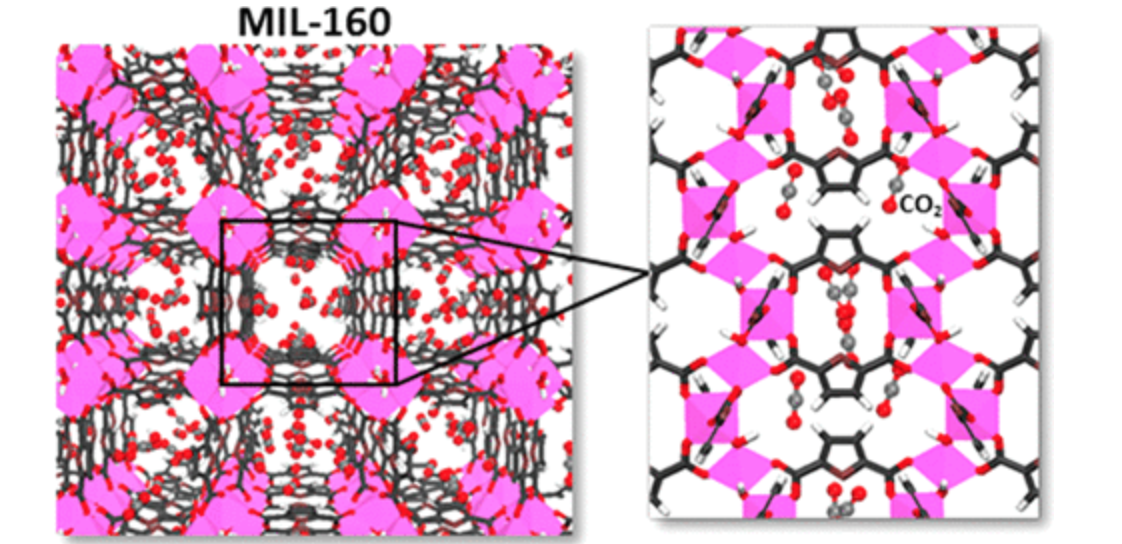
abstract = {One of the most promising technologies, with a low energy penalty, for CO2 capture from diverse gas mixtures is based on the adsorption process using adsorbents. Many efforts are still currently deployed to search for water stable porous metal–organic frameworks (MOFs) with high CO2 affinity combined with large CO2 uptake. In this context, we have selected the water stable and easily scalable Al-based MOF MIL-160 showing an ultramicroporosity and potential interacting sites (hydroxyl and furan), both features being a priori relevant to favor the selective adsorption of CO2 over other gases including H2, N2, CH4, and CO. Density functional theory (DFT) and force-field-based grand-canonical Monte Carlo (GCMC) simulations were first coupled to predict the strength of host/guest interactions and the adsorption isotherms for all guests as single components and binary mixtures. This computational approach reveals the promises of this solid for the selective adsorption of CO2 with respect to these other investigated gases, controlled by a combination of thermodynamics and confinement effects. These predicted performances were further supported by real-coadsorption measurements performed on shaped samples which indicated that MIL-160(Al) shows promising performance for the selective CO2 capture in post- and pre-combustion conditions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Borges, Daiane Damasceno; Maurin, Guillaume; Galvao, Douglas S
Design of Porous Metal-Organic Frameworks for Adsorption Driven Thermal Batteries Journal Article
Em: MRS Advances, vol. 2017, pp. 1-6, 2017.
@article{Borges2017b,
title = {Design of Porous Metal-Organic Frameworks for Adsorption Driven Thermal Batteries},
author = {Borges, Daiane Damasceno and Maurin, Guillaume and Galvao, Douglas S},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/design-of-porous-metalorganic-frameworks-for-adsorption-driven-thermal-batteries/A63B92E4D7E413D7CC047E152C7F22AF},
doi = {10.1557/adv.2017.181},
year = {2017},
date = {2017-02-15},
journal = {MRS Advances},
volume = {2017},
pages = {1-6},
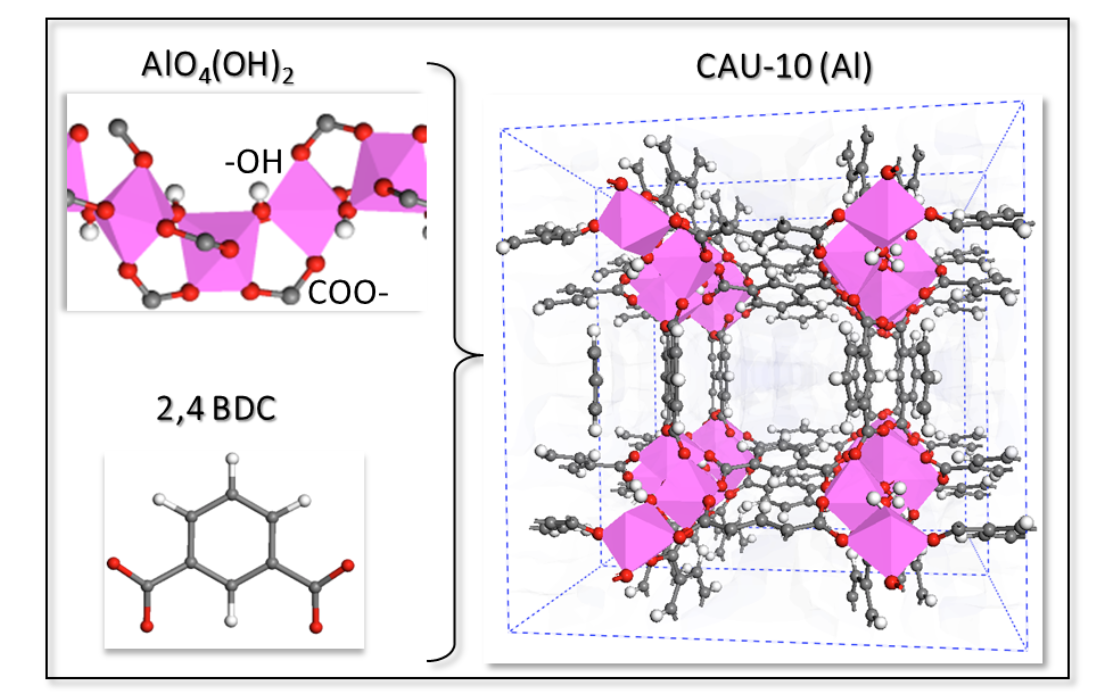
abstract = {Thermal batteries based on a reversible adsorption/desorption of a working fluid (water, methanol, ammonia) rather than the conventional vapor compression is a promising alternative to exploit waste thermal energy for heat reallocation. In this context, there is an increasing interest to find novel porous solids able to adsorb a high energy density of working fluid under low relative vapor pressure condition combined with an easy ability of regeneration (desorption) at low temperature, which are the major requirements for adsorption driven heat pumps and chillers. The porous crystalline hybrid materials named Metal–Organic Frameworks (MOF) represent a great source of inspiration for sorption based-applications owing to their tunable chemical and topological features associated with a large variability of pore sizes. Recently, we have designed a new MOF named MIL-160 (MIL stands for Materials of Institut Lavoisier), isostructural to CAU-10, built from the assembly of corner sharing aluminum chains octahedra AlO4(OH)2 with the 2,5-furandicarboxylic linker substituting the pristine organic linker, 1,4-benzenedicarboxylate. This ligand replacement strategy proved to enhance both the hydrophilicity of the MOF and its amount of water adsorbed at low p/p0. This designed solid was synthesized and its chemical stability/adsorption performances verified. Here, we have extended this study by incorporating other polar heterocyclic linkers and a comparative computational study of the water adsorption performances of these novel structures has been performed. To that purpose, the cell and geometry optimizations of all hypothetical frameworks were first performed at the density functional theory level and their water adsorption isotherms were further predicted by using force-field based Grand-Canonical Monte Carlo simulations. This study reveals the ease tunable water affinity of MOF for the desired application.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2017
BORGES, Daiane DAMASCENO; NORMAND, Perine; PERMIAKOVA, Anastasia; BABARAO, Ravichandar; HEYMANS, Nicolas; GALVAO, Douglas S.; SERRE, Christian; WEIRELD, Guy DE; MAURIN, Guillaume
Gas Adsorption and Separation by the Al-based Metal-Organic Framework MIL-160 Journal Article
Em: Journal of Physical Chemistry C, vol. 121, não 48, pp. 26822–26832, 2017.
Resumo | Links | BibTeX | Tags: MOFs, Simulation
@article{BORGES2017b,
title = {Gas Adsorption and Separation by the Al-based Metal-Organic Framework MIL-160},
author = {Daiane DAMASCENO BORGES and Perine NORMAND and Anastasia PERMIAKOVA and Ravichandar BABARAO and Nicolas HEYMANS and Douglas S. GALVAO and Christian SERRE and Guy DE WEIRELD and Guillaume MAURIN},
url = {http://pubs.acs.org/doi/abs/10.1021/acs.jpcc.7b08856},
doi = {DOI: 10.1021/acs.jpcc.7b08856},
year = {2017},
date = {2017-09-14},
journal = {Journal of Physical Chemistry C},
volume = {121},
number = {48},
pages = {26822–26832},
abstract = {One of the most promising technologies, with a low energy penalty, for CO2 capture from diverse gas mixtures is based on the adsorption process using adsorbents. Many efforts are still currently deployed to search for water stable porous metal–organic frameworks (MOFs) with high CO2 affinity combined with large CO2 uptake. In this context, we have selected the water stable and easily scalable Al-based MOF MIL-160 showing an ultramicroporosity and potential interacting sites (hydroxyl and furan), both features being a priori relevant to favor the selective adsorption of CO2 over other gases including H2, N2, CH4, and CO. Density functional theory (DFT) and force-field-based grand-canonical Monte Carlo (GCMC) simulations were first coupled to predict the strength of host/guest interactions and the adsorption isotherms for all guests as single components and binary mixtures. This computational approach reveals the promises of this solid for the selective adsorption of CO2 with respect to these other investigated gases, controlled by a combination of thermodynamics and confinement effects. These predicted performances were further supported by real-coadsorption measurements performed on shaped samples which indicated that MIL-160(Al) shows promising performance for the selective CO2 capture in post- and pre-combustion conditions.},
keywords = {MOFs, Simulation},
pubstate = {published},
tppubtype = {article}
}
Borges, Daiane Damasceno; Maurin, Guillaume; Galvao, Douglas S
Design of Porous Metal-Organic Frameworks for Adsorption Driven Thermal Batteries Journal Article
Em: MRS Advances, vol. 2017, pp. 1-6, 2017.
Resumo | Links | BibTeX | Tags: DFT, MOFs, thermal batteries
@article{Borges2017b,
title = {Design of Porous Metal-Organic Frameworks for Adsorption Driven Thermal Batteries},
author = {Borges, Daiane Damasceno and Maurin, Guillaume and Galvao, Douglas S},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/design-of-porous-metalorganic-frameworks-for-adsorption-driven-thermal-batteries/A63B92E4D7E413D7CC047E152C7F22AF},
doi = {10.1557/adv.2017.181},
year = {2017},
date = {2017-02-15},
journal = {MRS Advances},
volume = {2017},
pages = {1-6},
abstract = {Thermal batteries based on a reversible adsorption/desorption of a working fluid (water, methanol, ammonia) rather than the conventional vapor compression is a promising alternative to exploit waste thermal energy for heat reallocation. In this context, there is an increasing interest to find novel porous solids able to adsorb a high energy density of working fluid under low relative vapor pressure condition combined with an easy ability of regeneration (desorption) at low temperature, which are the major requirements for adsorption driven heat pumps and chillers. The porous crystalline hybrid materials named Metal–Organic Frameworks (MOF) represent a great source of inspiration for sorption based-applications owing to their tunable chemical and topological features associated with a large variability of pore sizes. Recently, we have designed a new MOF named MIL-160 (MIL stands for Materials of Institut Lavoisier), isostructural to CAU-10, built from the assembly of corner sharing aluminum chains octahedra AlO4(OH)2 with the 2,5-furandicarboxylic linker substituting the pristine organic linker, 1,4-benzenedicarboxylate. This ligand replacement strategy proved to enhance both the hydrophilicity of the MOF and its amount of water adsorbed at low p/p0. This designed solid was synthesized and its chemical stability/adsorption performances verified. Here, we have extended this study by incorporating other polar heterocyclic linkers and a comparative computational study of the water adsorption performances of these novel structures has been performed. To that purpose, the cell and geometry optimizations of all hypothetical frameworks were first performed at the density functional theory level and their water adsorption isotherms were further predicted by using force-field based Grand-Canonical Monte Carlo simulations. This study reveals the ease tunable water affinity of MOF for the desired application.
},
keywords = {DFT, MOFs, thermal batteries},
pubstate = {published},
tppubtype = {article}
}


