Solis, Daniel; Woellner, Cristiano F; Borges, Daiane D; Galvao, Douglas S
Mechanical and Thermal Stability of Graphyne and Graphdiyne Nanoscrolls Journal Article
In: MRS Advances, vol. 2017, pp. 129-134, 2017.
@article{Solis2017,
title = {Mechanical and Thermal Stability of Graphyne and Graphdiyne Nanoscrolls},
author = {Solis, Daniel and Woellner, Cristiano F and Borges, Daiane D and Galvao, Douglas S},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/mechanical-and-thermal-stability-of-graphyne-and-graphdiyne-nanoscrolls/202E7B7C471411200DE9D05C264726B8},
doi = {10.1557/adv.2017.130},
year = {2017},
date = {2017-02-01},
journal = {MRS Advances},
volume = {2017},
pages = {129-134},
abstract = {Graphynes and graphdiynes are carbon 2D allotrope structures presenting both sp2 and sp hybridized atoms. These materials have been theoretically predicted but due to intrinsic difficulties in their synthesis, only recently some of these structures have been experimentally realized. Graphyne nanoscrolls are structures obtained by rolling up graphyne sheets into papyrus-like structures. In this work, we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics of nanoscroll formation for a series of graphyne (α, β, and δ types) structures. We have also investigated their thermal stability for a temperature range of 200-1000K. Our results show that stable nanoscrolls can be formed for all structures considered here. Their stability depends on a critical value of the ratio between length and height of the graphyne sheets. Our findings also show that these structures are structurally less stable then graphene-based nanoscrolls. This can be explained by the graphyne higher structural porosity which results in a decreased pi-pi stacking interactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Machado, LD; Autreto, PAS; Galvao, DS
Graphyne Oxidation: Insights From a Reactive Molecular Dynamics Investigation Proceedings
Cambridge University Press, vol. 1549, 2013.
@proceedings{machado2013graphyne,
title = {Graphyne Oxidation: Insights From a Reactive Molecular Dynamics Investigation},
author = {Machado, LD and Autreto, PAS and Galvao, DS},
url = {http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8963025&fileId=S194642741300941X},
year = {2013},
date = {2013-01-01},
journal = {MRS Proceedings},
volume = {1549},
pages = {53--58},
publisher = {Cambridge University Press},
abstract = {Graphyne is a generic name for a family of carbon allotrope two-dimensional structures where sp2 (single and double bonds) and sp (triple bonds) hybridized states coexists. They exhibit very interesting electronic and mechanical properties sharing some of the unique graphene characteristics. Similarly to graphene, the graphyne electronic properties can be modified by chemical functionalization, such as; hydrogenation, fluorination and oxidation. Oxidation is of particular interest since it can produce significant structural damages.
In this work we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics and structural changes of the oxidation of single-layer graphyne membranes at room temperature. We have considered α, β, and γ-graphyne structures. Our results showed that the oxidation reactions are strongly site dependent and that the sp-hybridized carbon atoms are the preferential sites to chemical attacks. Our results also showed that the effectiveness of the oxidation (estimated from the number of oxygen atoms covalently bonded to carbon atoms) follows the α, β, γ-graphyne structure ordering. These differences can be explained by the fact that for α-graphyne structures the oxidation reactions occur in two steps: first, the oxygen atoms are trapped at the center of the large polygonal rings and then they react with the carbon atoms composing of the triple bonds. The small rings of γ-graphyne structures prevent these reactions to occur. The effectiveness of β-graphyne oxidation is between the α- and γ-graphynes.},
keywords = {},
pubstate = {published},
tppubtype = {proceedings}
}
In this work we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics and structural changes of the oxidation of single-layer graphyne membranes at room temperature. We have considered α, β, and γ-graphyne structures. Our results showed that the oxidation reactions are strongly site dependent and that the sp-hybridized carbon atoms are the preferential sites to chemical attacks. Our results also showed that the effectiveness of the oxidation (estimated from the number of oxygen atoms covalently bonded to carbon atoms) follows the α, β, γ-graphyne structure ordering. These differences can be explained by the fact that for α-graphyne structures the oxidation reactions occur in two steps: first, the oxygen atoms are trapped at the center of the large polygonal rings and then they react with the carbon atoms composing of the triple bonds. The small rings of γ-graphyne structures prevent these reactions to occur. The effectiveness of β-graphyne oxidation is between the α- and γ-graphynes.
2017
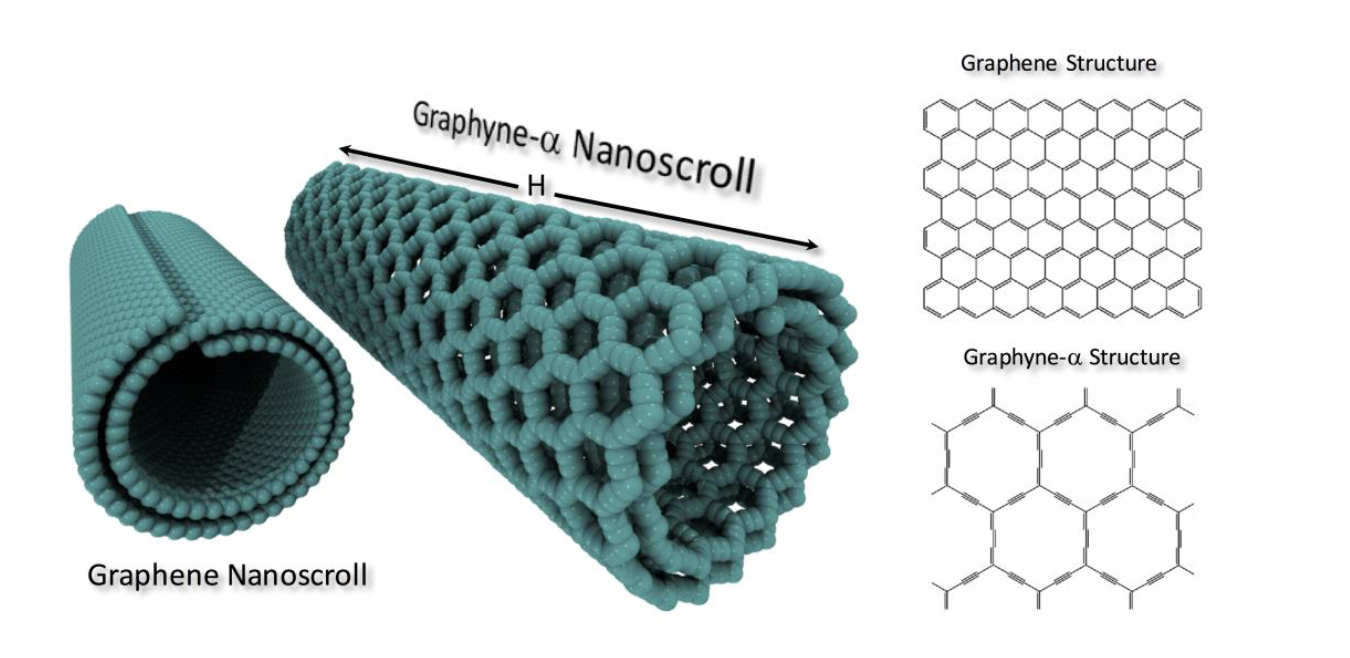
Solis, Daniel; Woellner, Cristiano F; Borges, Daiane D; Galvao, Douglas S
Mechanical and Thermal Stability of Graphyne and Graphdiyne Nanoscrolls Journal Article
In: MRS Advances, vol. 2017, pp. 129-134, 2017.
Abstract | Links | BibTeX | Tags: graphdiyne, Graphyne, Molecular Dynamics, Scrolls
@article{Solis2017,
title = {Mechanical and Thermal Stability of Graphyne and Graphdiyne Nanoscrolls},
author = {Solis, Daniel and Woellner, Cristiano F and Borges, Daiane D and Galvao, Douglas S},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/mechanical-and-thermal-stability-of-graphyne-and-graphdiyne-nanoscrolls/202E7B7C471411200DE9D05C264726B8},
doi = {10.1557/adv.2017.130},
year = {2017},
date = {2017-02-01},
journal = {MRS Advances},
volume = {2017},
pages = {129-134},
abstract = {Graphynes and graphdiynes are carbon 2D allotrope structures presenting both sp2 and sp hybridized atoms. These materials have been theoretically predicted but due to intrinsic difficulties in their synthesis, only recently some of these structures have been experimentally realized. Graphyne nanoscrolls are structures obtained by rolling up graphyne sheets into papyrus-like structures. In this work, we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics of nanoscroll formation for a series of graphyne (α, β, and δ types) structures. We have also investigated their thermal stability for a temperature range of 200-1000K. Our results show that stable nanoscrolls can be formed for all structures considered here. Their stability depends on a critical value of the ratio between length and height of the graphyne sheets. Our findings also show that these structures are structurally less stable then graphene-based nanoscrolls. This can be explained by the graphyne higher structural porosity which results in a decreased pi-pi stacking interactions.},
keywords = {graphdiyne, Graphyne, Molecular Dynamics, Scrolls},
pubstate = {published},
tppubtype = {article}
}
2013
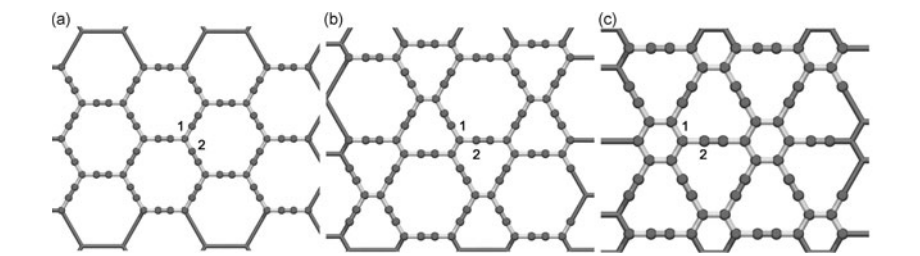
Machado, LD; Autreto, PAS; Galvao, DS
Graphyne Oxidation: Insights From a Reactive Molecular Dynamics Investigation Proceedings
Cambridge University Press, vol. 1549, 2013.
Abstract | Links | BibTeX | Tags: Graphdyine, Graphyne, Molecular Dynamics, Oxidation
@proceedings{machado2013graphyne,
title = {Graphyne Oxidation: Insights From a Reactive Molecular Dynamics Investigation},
author = {Machado, LD and Autreto, PAS and Galvao, DS},
url = {http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8963025&fileId=S194642741300941X},
year = {2013},
date = {2013-01-01},
journal = {MRS Proceedings},
volume = {1549},
pages = {53--58},
publisher = {Cambridge University Press},
abstract = {Graphyne is a generic name for a family of carbon allotrope two-dimensional structures where sp2 (single and double bonds) and sp (triple bonds) hybridized states coexists. They exhibit very interesting electronic and mechanical properties sharing some of the unique graphene characteristics. Similarly to graphene, the graphyne electronic properties can be modified by chemical functionalization, such as; hydrogenation, fluorination and oxidation. Oxidation is of particular interest since it can produce significant structural damages.
In this work we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics and structural changes of the oxidation of single-layer graphyne membranes at room temperature. We have considered α, β, and γ-graphyne structures. Our results showed that the oxidation reactions are strongly site dependent and that the sp-hybridized carbon atoms are the preferential sites to chemical attacks. Our results also showed that the effectiveness of the oxidation (estimated from the number of oxygen atoms covalently bonded to carbon atoms) follows the α, β, γ-graphyne structure ordering. These differences can be explained by the fact that for α-graphyne structures the oxidation reactions occur in two steps: first, the oxygen atoms are trapped at the center of the large polygonal rings and then they react with the carbon atoms composing of the triple bonds. The small rings of γ-graphyne structures prevent these reactions to occur. The effectiveness of β-graphyne oxidation is between the α- and γ-graphynes.},
keywords = {Graphdyine, Graphyne, Molecular Dynamics, Oxidation},
pubstate = {published},
tppubtype = {proceedings}
}
In this work we have investigated, through fully atomistic reactive molecular dynamics simulations, the dynamics and structural changes of the oxidation of single-layer graphyne membranes at room temperature. We have considered α, β, and γ-graphyne structures. Our results showed that the oxidation reactions are strongly site dependent and that the sp-hybridized carbon atoms are the preferential sites to chemical attacks. Our results also showed that the effectiveness of the oxidation (estimated from the number of oxygen atoms covalently bonded to carbon atoms) follows the α, β, γ-graphyne structure ordering. These differences can be explained by the fact that for α-graphyne structures the oxidation reactions occur in two steps: first, the oxygen atoms are trapped at the center of the large polygonal rings and then they react with the carbon atoms composing of the triple bonds. The small rings of γ-graphyne structures prevent these reactions to occur. The effectiveness of β-graphyne oxidation is between the α- and γ-graphynes.
http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ


