1.
Dos Santos, SG; Pires, MS; Lemos, V; Freire, VN; Caetano, EWS; Galvao, DS; Sato, F; Albuquerque, EL
C60-derived nanobaskets: stability, vibrational signatures, and molecular trapping Journal Article
In: Nanotechnology, vol. 20, no. 39, pp. 395701, 2009.
@article{dos2009c60,
title = {C60-derived nanobaskets: stability, vibrational signatures, and molecular trapping},
author = {Dos Santos, SG and Pires, MS and Lemos, V and Freire, VN and Caetano, EWS and Galvao, DS and Sato, F and Albuquerque, EL},
url = {http://iopscience.iop.org/0957-4484/20/39/395701},
year = {2009},
date = {2009-01-01},
journal = {Nanotechnology},
volume = {20},
number = {39},
pages = {395701},
publisher = {IOP Publishing},
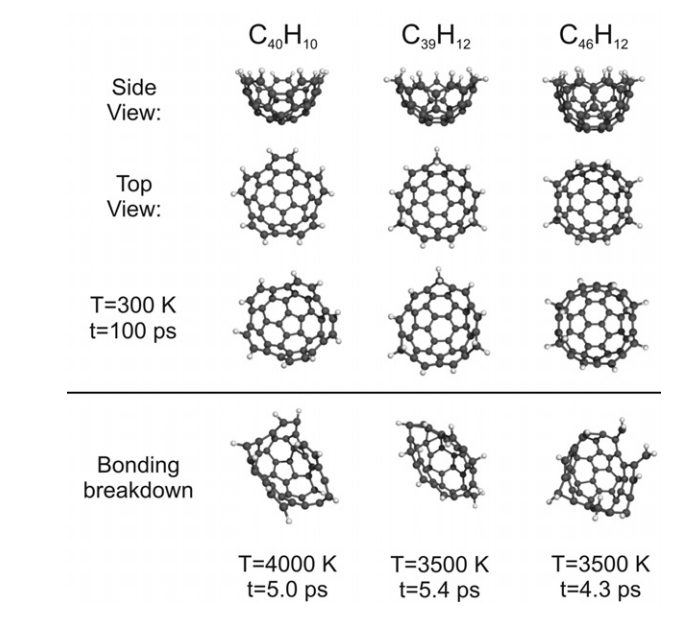
abstract = {C60-derived nanobaskets, with chemical formulae (symmetry point group) C40H10 (C5v), C39H12 (C3v), C46H12 (C2v), were investigated. Molecular dynamic simulations (MDSs) indicate that the molecules preserve their bonding frame for temperatures up to 300 K (simulation time 100 ps), and maintain atomic cohesion for at least 4 ps at temperatures up to 3500 K. The infrared spectra of the C60-derived nanobaskets were simulated through density functional theory (DFT) calculations, allowing for the attribution of infrared signatures specific to each carbon nanobasket. The possibility of using C60-derived nanobaskets as molecular containers is demonstrated by performing a DFT study of their bonding to hydrogen, water, and L-alanine. The carbon nanostructures presented here show a higher bonding energy (~1.0 eV), suggesting that a family of nanostructures, Cn-derived (n = 60,70,76,80, etc) nanobaskets, could work as molecular containers, paving the way for future developments such as tunable traps for complex molecular systems.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
C60-derived nanobaskets, with chemical formulae (symmetry point group) C40H10 (C5v), C39H12 (C3v), C46H12 (C2v), were investigated. Molecular dynamic simulations (MDSs) indicate that the molecules preserve their bonding frame for temperatures up to 300 K (simulation time 100 ps), and maintain atomic cohesion for at least 4 ps at temperatures up to 3500 K. The infrared spectra of the C60-derived nanobaskets were simulated through density functional theory (DFT) calculations, allowing for the attribution of infrared signatures specific to each carbon nanobasket. The possibility of using C60-derived nanobaskets as molecular containers is demonstrated by performing a DFT study of their bonding to hydrogen, water, and L-alanine. The carbon nanostructures presented here show a higher bonding energy (~1.0 eV), suggesting that a family of nanostructures, Cn-derived (n = 60,70,76,80, etc) nanobaskets, could work as molecular containers, paving the way for future developments such as tunable traps for complex molecular systems.
2009
1.
Dos Santos, SG; Pires, MS; Lemos, V; Freire, VN; Caetano, EWS; Galvao, DS; Sato, F; Albuquerque, EL
C60-derived nanobaskets: stability, vibrational signatures, and molecular trapping Journal Article
In: Nanotechnology, vol. 20, no. 39, pp. 395701, 2009.
Abstract | Links | BibTeX | Tags: Fullerenes, Molecular Dynamics, nanobaskets, nanobowls
@article{dos2009c60,
title = {C60-derived nanobaskets: stability, vibrational signatures, and molecular trapping},
author = {Dos Santos, SG and Pires, MS and Lemos, V and Freire, VN and Caetano, EWS and Galvao, DS and Sato, F and Albuquerque, EL},
url = {http://iopscience.iop.org/0957-4484/20/39/395701},
year = {2009},
date = {2009-01-01},
journal = {Nanotechnology},
volume = {20},
number = {39},
pages = {395701},
publisher = {IOP Publishing},
abstract = {C60-derived nanobaskets, with chemical formulae (symmetry point group) C40H10 (C5v), C39H12 (C3v), C46H12 (C2v), were investigated. Molecular dynamic simulations (MDSs) indicate that the molecules preserve their bonding frame for temperatures up to 300 K (simulation time 100 ps), and maintain atomic cohesion for at least 4 ps at temperatures up to 3500 K. The infrared spectra of the C60-derived nanobaskets were simulated through density functional theory (DFT) calculations, allowing for the attribution of infrared signatures specific to each carbon nanobasket. The possibility of using C60-derived nanobaskets as molecular containers is demonstrated by performing a DFT study of their bonding to hydrogen, water, and L-alanine. The carbon nanostructures presented here show a higher bonding energy (~1.0 eV), suggesting that a family of nanostructures, Cn-derived (n = 60,70,76,80, etc) nanobaskets, could work as molecular containers, paving the way for future developments such as tunable traps for complex molecular systems.},
keywords = {Fullerenes, Molecular Dynamics, nanobaskets, nanobowls},
pubstate = {published},
tppubtype = {article}
}
C60-derived nanobaskets, with chemical formulae (symmetry point group) C40H10 (C5v), C39H12 (C3v), C46H12 (C2v), were investigated. Molecular dynamic simulations (MDSs) indicate that the molecules preserve their bonding frame for temperatures up to 300 K (simulation time 100 ps), and maintain atomic cohesion for at least 4 ps at temperatures up to 3500 K. The infrared spectra of the C60-derived nanobaskets were simulated through density functional theory (DFT) calculations, allowing for the attribution of infrared signatures specific to each carbon nanobasket. The possibility of using C60-derived nanobaskets as molecular containers is demonstrated by performing a DFT study of their bonding to hydrogen, water, and L-alanine. The carbon nanostructures presented here show a higher bonding energy (~1.0 eV), suggesting that a family of nanostructures, Cn-derived (n = 60,70,76,80, etc) nanobaskets, could work as molecular containers, paving the way for future developments such as tunable traps for complex molecular systems.
http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ


