http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ
Sato, Fernando; Braga, Scheila F; Santos, Helio F dos; Galvao, Douglas S
Structure-Activity Relationship Investigation of Some New Tetracyclines by Electronic Index Methodology Journal Article
Em: arXiv preprint arXiv:0708.2931, 2007.
@article{sato2007structure,
title = {Structure-Activity Relationship Investigation of Some New Tetracyclines by Electronic Index Methodology},
author = {Sato, Fernando and Braga, Scheila F and Santos, Helio F dos and Galvao, Douglas S},
url = {http://arxiv.org/abs/0708.2931},
year = {2007},
date = {2007-01-01},
journal = {arXiv preprint arXiv:0708.2931},
abstract = {Tetracyclines are an old class of molecules that constitute a broad-spectrum antibiotics. Since the first member of tetracycline family were isolated, the clinical importance of these compounds as therapeutic and prophylactic agents against a wide range of infections has stimulated efforts to define their mode of action as inhibitors of bacterial reproduction. We used three SAR methodologies for the analysis of biological activity of a set of 104 tetracycline compounds. Our calculation were carried out using the semi-empirical Austin Method One (AM1) and Parametric Method 3 (PM3). Electronic Indices Methodology (EIM), Principal Component Analysis (PCA) and Artificial Neural Networks (ANN) were applied to the classification of 14 old and 90 new proposed derivatives of tetracyclines. Our results make evident the importance of EIM descriptors in pattern recognition and also show that the EIM can be effectively used to predict the biological activity of Tetracyclines.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Troche, Karla S; Braga, Scheila F; Coluci, Vitor R; Galvao, Douglas S
Carcinogenic classification of polycyclic aromatic hydrocarbons through theoretical descriptors Journal Article
Em: International journal of quantum chemistry, vol. 103, não 5, pp. 718–730, 2005.
@article{troche2005carcinogenic,
title = {Carcinogenic classification of polycyclic aromatic hydrocarbons through theoretical descriptors},
author = {Troche, Karla S and Braga, Scheila F and Coluci, Vitor R and Galvao, Douglas S},
url = {http://onlinelibrary.wiley.com/doi/10.1002/qua.20529/full},
year = {2005},
date = {2005-01-01},
journal = {International journal of quantum chemistry},
volume = {103},
number = {5},
pages = {718--730},
publisher = {Wiley Online Library},
abstract = {Polycyclic aromatic hydrocarbons (PAHs) constitute an importantfamily of molecules capable of inducing chemical carcinogenesis. In this work we reporta comparative structure–activity relationship (SAR) study for 81 PAHs using differentmethodologies. The recently developed electronic indices methodology (EIM) withquantum descriptors obtained from different semiempirical methods (AM1, PM3, andPM5) was contrasted against more standard pattern recognition methods (PRMs),principal component analysis (PCA), hierarchical cluster analysis (HCA), Kth nearestneighbor (KNN), soft independent modeling of class analogies (SIMCA), and neuralnetworks (NN). Our results show that PRMs validate the statistical value of electronicparameters derived from EIM analysis and their ability to identify active compounds.EIM outperformed more standard SAR methodologies and does not appear to besignificantly Hamiltonian-dependent.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Coluci, Vitor Rafael; Vendrame, Rosana; Braga, RS; Galvao, DS
Em: Journal of chemical information and computer sciences, vol. 42, não 6, pp. 1479–1489, 2002.
@article{coluci2002identifying,
title = {Identifying relevant molecular descriptors related to carcinogenic activity of polycyclic aromatic hydrocarbons (PAHs) using pattern recognition methods},
author = {Coluci, Vitor Rafael and Vendrame, Rosana and Braga, RS and Galvao, DS},
url = {http://pubs.acs.org/doi/abs/10.1021/ci025577%2B},
year = {2002},
date = {2002-01-01},
journal = {Journal of chemical information and computer sciences},
volume = {42},
number = {6},
pages = {1479--1489},
publisher = {American Chemical Society},
abstract = {Polycyclic Aromatic Hydrocarbons (PAHs) constitute an important family of molecules capable of inducing
chemical carcinogenesis. In this work we report structure-activity relationship (SAR) studies for 81 PAHs
using the pattern-recognition methods Principal Component Analysis (PCA), Hierarchical Clustering Analysis
(HCA) and Neural Networks (NN). The used molecular descriptors were obtained from the semiempirical
Parametric Method 3 (PM3) calculations. We have developed a new procedure that is capable of identifying
the PAHs’ carcinogenic activity with an accuracy higher than 80%. PCA selected molecular descriptors
that can be directly correlated with some models proposed to PAHs’ metabolic activation mechanism leading
to the formation of PAHs-DNA adducts. PCA, HCA and NN validate the energy separation between the
highest occupied molecular orbital and its next lower level as a major descriptor defining the carcinogenic
activity. This descriptor has been only recently discussed in the literature as one new possible universal
parameter for defining the biological activity of several classes of compounds.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
chemical carcinogenesis. In this work we report structure-activity relationship (SAR) studies for 81 PAHs
using the pattern-recognition methods Principal Component Analysis (PCA), Hierarchical Clustering Analysis
(HCA) and Neural Networks (NN). The used molecular descriptors were obtained from the semiempirical
Parametric Method 3 (PM3) calculations. We have developed a new procedure that is capable of identifying
the PAHs’ carcinogenic activity with an accuracy higher than 80%. PCA selected molecular descriptors
that can be directly correlated with some models proposed to PAHs’ metabolic activation mechanism leading
to the formation of PAHs-DNA adducts. PCA, HCA and NN validate the energy separation between the
highest occupied molecular orbital and its next lower level as a major descriptor defining the carcinogenic
activity. This descriptor has been only recently discussed in the literature as one new possible universal
parameter for defining the biological activity of several classes of compounds.
Vendrame, R; Coluci, VR; Braga, RS; Galvao, DS
Structure--activity relationship (SAR) studies of the tripos benchmark steroids Journal Article
Em: Journal of Molecular Structure: THEOCHEM, vol. 619, não 1, pp. 195–205, 2002.
@article{vendrame2002structure,
title = {Structure--activity relationship (SAR) studies of the tripos benchmark steroids},
author = {Vendrame, R and Coluci, VR and Braga, RS and Galvao, DS},
url = {http://www.sciencedirect.com/science/article/pii/S016612800200578X},
year = {2002},
date = {2002-01-01},
journal = {Journal of Molecular Structure: THEOCHEM},
volume = {619},
number = {1},
pages = {195--205},
publisher = {Elsevier},
abstract = {We report here qualitative structure–activity relationship (SAR) studies for the molecular set called Tripos or Cramer steroid data set. These compounds are known to bind to corticosteroid binding globulin (CBG). In the present work we have used the electronic indices methodology (EIM). The EIM is based on Boolean relational rules exploring the concepts of local density of states and critical values for energy separation involving frontier orbitals. We have also carried out comparative principal component analysis (PCA) and hierarchical clustering analysis (HCA) studies with molecular descriptors obtained from EIM calculations. EIM, PCA and HCA correctly predict (100% accuracy) the steroid's biological activity. The present studies reinforce the universal applicability of the EIM descriptors and show that the combined use of EIM coupled to PCA can be a new efficient and powerful SAR tool.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2007
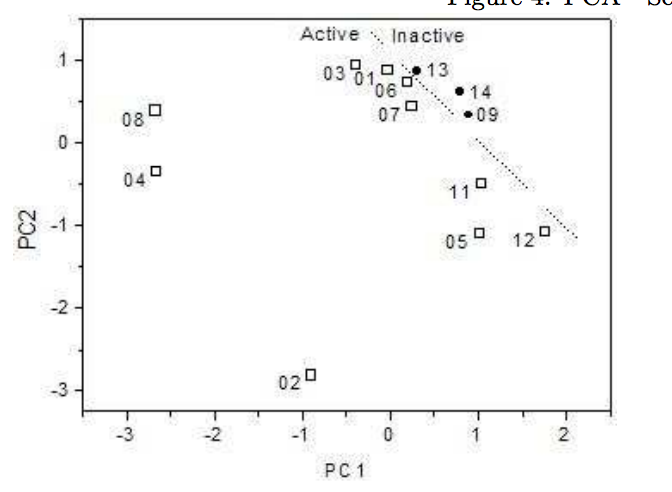
Sato, Fernando; Braga, Scheila F; Santos, Helio F dos; Galvao, Douglas S
Structure-Activity Relationship Investigation of Some New Tetracyclines by Electronic Index Methodology Journal Article
Em: arXiv preprint arXiv:0708.2931, 2007.
Resumo | Links | BibTeX | Tags: Drug Design, Electronic Structure, Neural Networks, PCA/HCA, Tetracyclines, Theory of Electronic Indices
@article{sato2007structure,
title = {Structure-Activity Relationship Investigation of Some New Tetracyclines by Electronic Index Methodology},
author = {Sato, Fernando and Braga, Scheila F and Santos, Helio F dos and Galvao, Douglas S},
url = {http://arxiv.org/abs/0708.2931},
year = {2007},
date = {2007-01-01},
journal = {arXiv preprint arXiv:0708.2931},
abstract = {Tetracyclines are an old class of molecules that constitute a broad-spectrum antibiotics. Since the first member of tetracycline family were isolated, the clinical importance of these compounds as therapeutic and prophylactic agents against a wide range of infections has stimulated efforts to define their mode of action as inhibitors of bacterial reproduction. We used three SAR methodologies for the analysis of biological activity of a set of 104 tetracycline compounds. Our calculation were carried out using the semi-empirical Austin Method One (AM1) and Parametric Method 3 (PM3). Electronic Indices Methodology (EIM), Principal Component Analysis (PCA) and Artificial Neural Networks (ANN) were applied to the classification of 14 old and 90 new proposed derivatives of tetracyclines. Our results make evident the importance of EIM descriptors in pattern recognition and also show that the EIM can be effectively used to predict the biological activity of Tetracyclines.},
keywords = {Drug Design, Electronic Structure, Neural Networks, PCA/HCA, Tetracyclines, Theory of Electronic Indices},
pubstate = {published},
tppubtype = {article}
}
2005
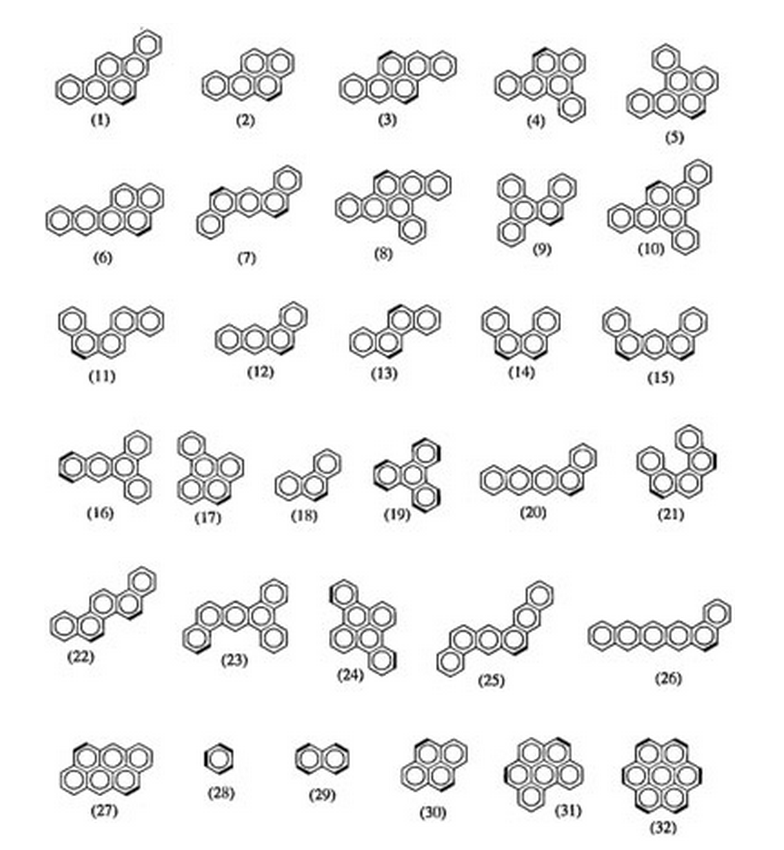
Troche, Karla S; Braga, Scheila F; Coluci, Vitor R; Galvao, Douglas S
Carcinogenic classification of polycyclic aromatic hydrocarbons through theoretical descriptors Journal Article
Em: International journal of quantum chemistry, vol. 103, não 5, pp. 718–730, 2005.
Resumo | Links | BibTeX | Tags: Carcinogenesis, HCA, Neural Networks, PCA, Polycyclic Aromatic Hydrocarbons (PAHs), Theory of Electronic Indices
@article{troche2005carcinogenic,
title = {Carcinogenic classification of polycyclic aromatic hydrocarbons through theoretical descriptors},
author = {Troche, Karla S and Braga, Scheila F and Coluci, Vitor R and Galvao, Douglas S},
url = {http://onlinelibrary.wiley.com/doi/10.1002/qua.20529/full},
year = {2005},
date = {2005-01-01},
journal = {International journal of quantum chemistry},
volume = {103},
number = {5},
pages = {718--730},
publisher = {Wiley Online Library},
abstract = {Polycyclic aromatic hydrocarbons (PAHs) constitute an importantfamily of molecules capable of inducing chemical carcinogenesis. In this work we reporta comparative structure–activity relationship (SAR) study for 81 PAHs using differentmethodologies. The recently developed electronic indices methodology (EIM) withquantum descriptors obtained from different semiempirical methods (AM1, PM3, andPM5) was contrasted against more standard pattern recognition methods (PRMs),principal component analysis (PCA), hierarchical cluster analysis (HCA), Kth nearestneighbor (KNN), soft independent modeling of class analogies (SIMCA), and neuralnetworks (NN). Our results show that PRMs validate the statistical value of electronicparameters derived from EIM analysis and their ability to identify active compounds.EIM outperformed more standard SAR methodologies and does not appear to besignificantly Hamiltonian-dependent.},
keywords = {Carcinogenesis, HCA, Neural Networks, PCA, Polycyclic Aromatic Hydrocarbons (PAHs), Theory of Electronic Indices},
pubstate = {published},
tppubtype = {article}
}
2002
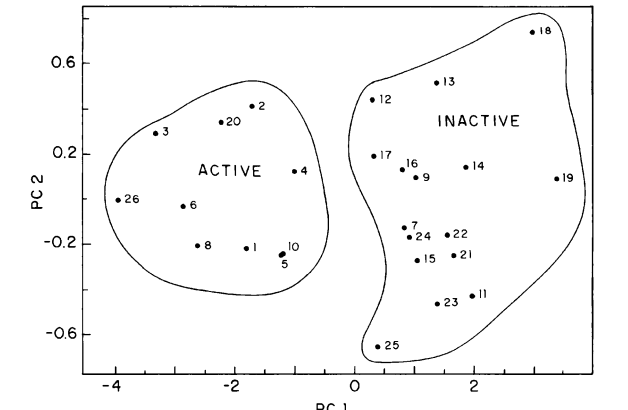
Coluci, Vitor Rafael; Vendrame, Rosana; Braga, RS; Galvao, DS
Em: Journal of chemical information and computer sciences, vol. 42, não 6, pp. 1479–1489, 2002.
Resumo | Links | BibTeX | Tags: Carcinogenesis, Neural Networks, PCA/HCA, Polycyclic Aromatic Hydrocarbons (PAHs), Theory of Electronic Indices
@article{coluci2002identifying,
title = {Identifying relevant molecular descriptors related to carcinogenic activity of polycyclic aromatic hydrocarbons (PAHs) using pattern recognition methods},
author = {Coluci, Vitor Rafael and Vendrame, Rosana and Braga, RS and Galvao, DS},
url = {http://pubs.acs.org/doi/abs/10.1021/ci025577%2B},
year = {2002},
date = {2002-01-01},
journal = {Journal of chemical information and computer sciences},
volume = {42},
number = {6},
pages = {1479--1489},
publisher = {American Chemical Society},
abstract = {Polycyclic Aromatic Hydrocarbons (PAHs) constitute an important family of molecules capable of inducing
chemical carcinogenesis. In this work we report structure-activity relationship (SAR) studies for 81 PAHs
using the pattern-recognition methods Principal Component Analysis (PCA), Hierarchical Clustering Analysis
(HCA) and Neural Networks (NN). The used molecular descriptors were obtained from the semiempirical
Parametric Method 3 (PM3) calculations. We have developed a new procedure that is capable of identifying
the PAHs’ carcinogenic activity with an accuracy higher than 80%. PCA selected molecular descriptors
that can be directly correlated with some models proposed to PAHs’ metabolic activation mechanism leading
to the formation of PAHs-DNA adducts. PCA, HCA and NN validate the energy separation between the
highest occupied molecular orbital and its next lower level as a major descriptor defining the carcinogenic
activity. This descriptor has been only recently discussed in the literature as one new possible universal
parameter for defining the biological activity of several classes of compounds.},
keywords = {Carcinogenesis, Neural Networks, PCA/HCA, Polycyclic Aromatic Hydrocarbons (PAHs), Theory of Electronic Indices},
pubstate = {published},
tppubtype = {article}
}
chemical carcinogenesis. In this work we report structure-activity relationship (SAR) studies for 81 PAHs
using the pattern-recognition methods Principal Component Analysis (PCA), Hierarchical Clustering Analysis
(HCA) and Neural Networks (NN). The used molecular descriptors were obtained from the semiempirical
Parametric Method 3 (PM3) calculations. We have developed a new procedure that is capable of identifying
the PAHs’ carcinogenic activity with an accuracy higher than 80%. PCA selected molecular descriptors
that can be directly correlated with some models proposed to PAHs’ metabolic activation mechanism leading
to the formation of PAHs-DNA adducts. PCA, HCA and NN validate the energy separation between the
highest occupied molecular orbital and its next lower level as a major descriptor defining the carcinogenic
activity. This descriptor has been only recently discussed in the literature as one new possible universal
parameter for defining the biological activity of several classes of compounds.
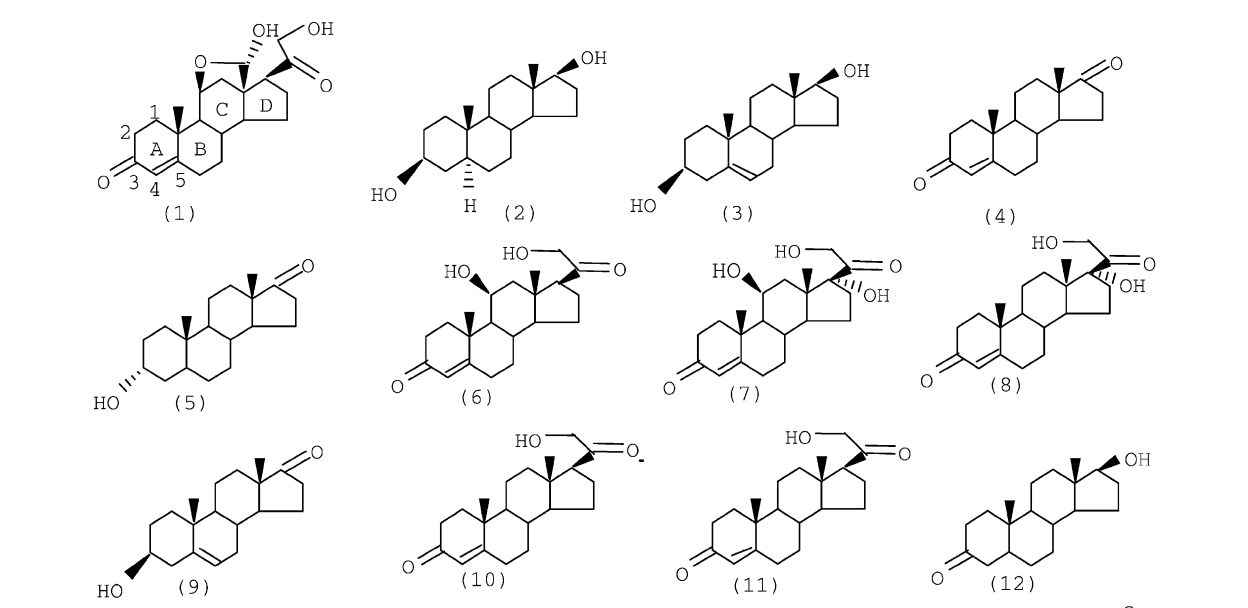
Vendrame, R; Coluci, VR; Braga, RS; Galvao, DS
Structure--activity relationship (SAR) studies of the tripos benchmark steroids Journal Article
Em: Journal of Molecular Structure: THEOCHEM, vol. 619, não 1, pp. 195–205, 2002.
Resumo | Links | BibTeX | Tags: Drug Design, Electronic Structure, HCA/PCA, Neural Networks, Tripos
@article{vendrame2002structure,
title = {Structure--activity relationship (SAR) studies of the tripos benchmark steroids},
author = {Vendrame, R and Coluci, VR and Braga, RS and Galvao, DS},
url = {http://www.sciencedirect.com/science/article/pii/S016612800200578X},
year = {2002},
date = {2002-01-01},
journal = {Journal of Molecular Structure: THEOCHEM},
volume = {619},
number = {1},
pages = {195--205},
publisher = {Elsevier},
abstract = {We report here qualitative structure–activity relationship (SAR) studies for the molecular set called Tripos or Cramer steroid data set. These compounds are known to bind to corticosteroid binding globulin (CBG). In the present work we have used the electronic indices methodology (EIM). The EIM is based on Boolean relational rules exploring the concepts of local density of states and critical values for energy separation involving frontier orbitals. We have also carried out comparative principal component analysis (PCA) and hierarchical clustering analysis (HCA) studies with molecular descriptors obtained from EIM calculations. EIM, PCA and HCA correctly predict (100% accuracy) the steroid's biological activity. The present studies reinforce the universal applicability of the EIM descriptors and show that the combined use of EIM coupled to PCA can be a new efficient and powerful SAR tool.
},
keywords = {Drug Design, Electronic Structure, HCA/PCA, Neural Networks, Tripos},
pubstate = {published},
tppubtype = {article}
}


