1.
Giro, R; Galvao, DS
Semiempirical studies of the electronic structure of polyphenylene sulfide phenyleneamine Journal Article
In: International journal of quantum chemistry, vol. 95, no. 3, pp. 252–259, 2003.
@article{giro2003semiempirical,
title = {Semiempirical studies of the electronic structure of polyphenylene sulfide phenyleneamine},
author = {Giro, R and Galvao, DS},
url = {http://onlinelibrary.wiley.com/doi/10.1002/qua.10722/full},
year = {2003},
date = {2003-01-01},
journal = {International journal of quantum chemistry},
volume = {95},
number = {3},
pages = {252--259},
publisher = {Wiley Subscription Services, Inc., A Wiley Company},
abstract = {Polyphenylene sulfide (PPS) and polyaniline (PANI) are heteroatom-containing polymers with unique properties. While PPS provides a good level of chemical and thermal stability, PANI is important due to its ability to form electrically conducting films. A combination of PPS and PANI, with alternating phenyleneamine and phenylene sulfide blocks, resulted in a new material combining the structural features of PPS and PANI. This copolymer is known as polyphenylene sulfide–phenyleneamine (PPSA). In this work we present geometric and spectroscopic studies on PPSA oligomers using the well-known semiempirical methods PM3 (parametric method 3) and ZINDO-S/CI (Zerner's intermediate neglect of differential overlap/spectroscopic-configuration interaction). PM3 results show that long PPSA oligomers present alternating in- and out-of-plane rings with torsion angles about 60°. The ZINDO-simulated spectra compare well with the available experimental data. The origin of the low PPSA conductivity is addressed in terms of electronic features presented by isolated polymeric chains. © 2003 Wiley Periodicals, Inc. Int J Quantum Chem 95: 252–259, 2003},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
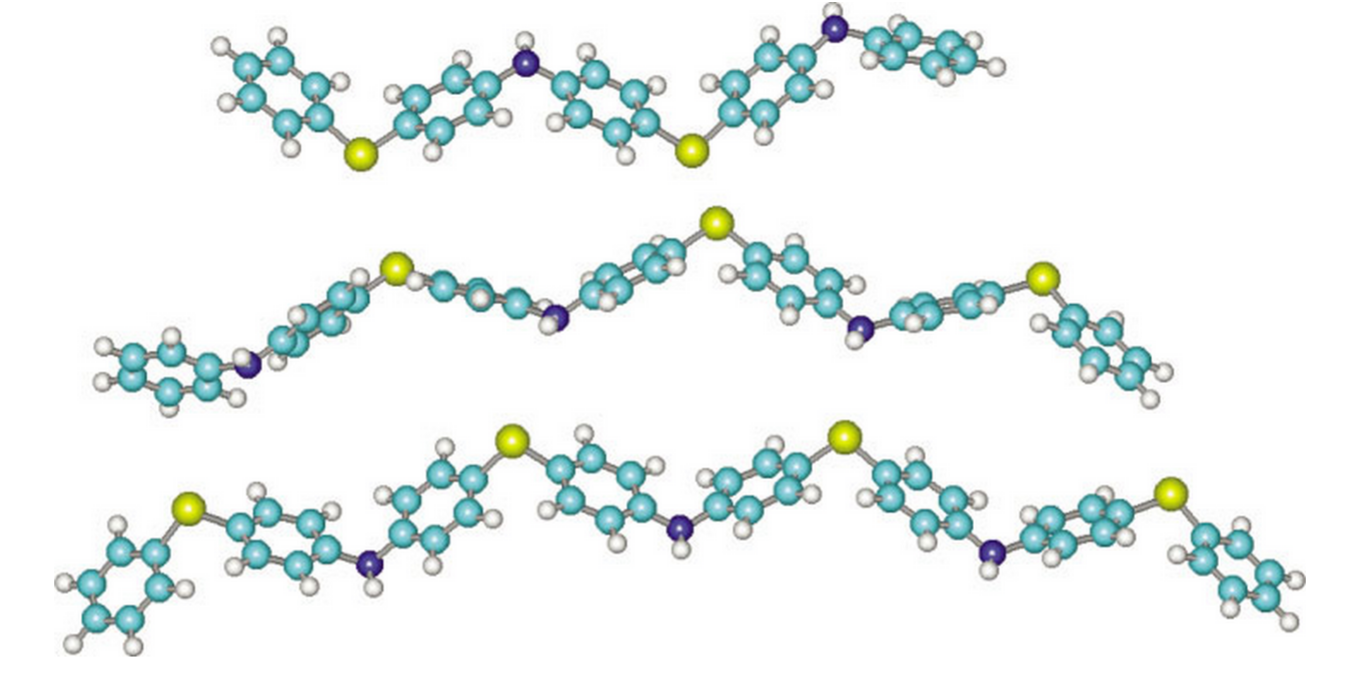
Polyphenylene sulfide (PPS) and polyaniline (PANI) are heteroatom-containing polymers with unique properties. While PPS provides a good level of chemical and thermal stability, PANI is important due to its ability to form electrically conducting films. A combination of PPS and PANI, with alternating phenyleneamine and phenylene sulfide blocks, resulted in a new material combining the structural features of PPS and PANI. This copolymer is known as polyphenylene sulfide–phenyleneamine (PPSA). In this work we present geometric and spectroscopic studies on PPSA oligomers using the well-known semiempirical methods PM3 (parametric method 3) and ZINDO-S/CI (Zerner's intermediate neglect of differential overlap/spectroscopic-configuration interaction). PM3 results show that long PPSA oligomers present alternating in- and out-of-plane rings with torsion angles about 60°. The ZINDO-simulated spectra compare well with the available experimental data. The origin of the low PPSA conductivity is addressed in terms of electronic features presented by isolated polymeric chains. © 2003 Wiley Periodicals, Inc. Int J Quantum Chem 95: 252–259, 2003
2003
1.
Giro, R; Galvao, DS
Semiempirical studies of the electronic structure of polyphenylene sulfide phenyleneamine Journal Article
In: International journal of quantum chemistry, vol. 95, no. 3, pp. 252–259, 2003.
Abstract | Links | BibTeX | Tags: co-polymers, Conducting Polymers, Electronic Structure, PANi, PPS, PPSA
@article{giro2003semiempirical,
title = {Semiempirical studies of the electronic structure of polyphenylene sulfide phenyleneamine},
author = {Giro, R and Galvao, DS},
url = {http://onlinelibrary.wiley.com/doi/10.1002/qua.10722/full},
year = {2003},
date = {2003-01-01},
journal = {International journal of quantum chemistry},
volume = {95},
number = {3},
pages = {252--259},
publisher = {Wiley Subscription Services, Inc., A Wiley Company},
abstract = {Polyphenylene sulfide (PPS) and polyaniline (PANI) are heteroatom-containing polymers with unique properties. While PPS provides a good level of chemical and thermal stability, PANI is important due to its ability to form electrically conducting films. A combination of PPS and PANI, with alternating phenyleneamine and phenylene sulfide blocks, resulted in a new material combining the structural features of PPS and PANI. This copolymer is known as polyphenylene sulfide–phenyleneamine (PPSA). In this work we present geometric and spectroscopic studies on PPSA oligomers using the well-known semiempirical methods PM3 (parametric method 3) and ZINDO-S/CI (Zerner's intermediate neglect of differential overlap/spectroscopic-configuration interaction). PM3 results show that long PPSA oligomers present alternating in- and out-of-plane rings with torsion angles about 60°. The ZINDO-simulated spectra compare well with the available experimental data. The origin of the low PPSA conductivity is addressed in terms of electronic features presented by isolated polymeric chains. © 2003 Wiley Periodicals, Inc. Int J Quantum Chem 95: 252–259, 2003},
keywords = {co-polymers, Conducting Polymers, Electronic Structure, PANi, PPS, PPSA},
pubstate = {published},
tppubtype = {article}
}
Polyphenylene sulfide (PPS) and polyaniline (PANI) are heteroatom-containing polymers with unique properties. While PPS provides a good level of chemical and thermal stability, PANI is important due to its ability to form electrically conducting films. A combination of PPS and PANI, with alternating phenyleneamine and phenylene sulfide blocks, resulted in a new material combining the structural features of PPS and PANI. This copolymer is known as polyphenylene sulfide–phenyleneamine (PPSA). In this work we present geometric and spectroscopic studies on PPSA oligomers using the well-known semiempirical methods PM3 (parametric method 3) and ZINDO-S/CI (Zerner's intermediate neglect of differential overlap/spectroscopic-configuration interaction). PM3 results show that long PPSA oligomers present alternating in- and out-of-plane rings with torsion angles about 60°. The ZINDO-simulated spectra compare well with the available experimental data. The origin of the low PPSA conductivity is addressed in terms of electronic features presented by isolated polymeric chains. © 2003 Wiley Periodicals, Inc. Int J Quantum Chem 95: 252–259, 2003
http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ


