Borges, Daiane Damasceno; Woellner, Cristiano F.; Autreto, Pedro A. S.; Galvao, Douglas S.
Water/alcohol separation via layered oxide graphene membranes Journal Article
In: MRS Advances, vol. 3, no. 1-2, pp. 109-114, 2018.
@article{Borges2018d,
title = {Water/alcohol separation via layered oxide graphene membranes},
author = {Daiane Damasceno Borges and Cristiano F. Woellner and Pedro A. S. Autreto and Douglas S. Galvao},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/wateralcohol-separation-in-graphene-oxide-membranes-insights-from-molecular-dynamics-and-monte-carlo-simulations/C61C66FF48D35EB2DB3408ACCE96C41A},
doi = { https://doi.org/10.1557/adv.2018.192},
year = {2018},
date = {2018-02-13},
journal = {MRS Advances},
volume = {3},
number = {1-2},
pages = {109-114},
abstract = {Graphene-based membranes have been investigated as promising candidates for water filtration and gas separation applications. Experimental evidences have shown that graphene oxide can be impermeable to liquids, vapors and gases, while allowing a fast permeation of water molecules. This phenomenon has been attributed to the formation of a network of nano capillaries that allow nearly frictionless water flow while blocking other molecules by steric hindrance effects. It is supposed that water molecules are transported through the percolated two-dimensional channels formed between graphene-based sheets. Although these channels allow fast water permeation in such materials, the flow rates are strongly dependent on how the membranes are fabricated. Also, some fundamental issues regarding the nanoscale mechanisms of water permeation are still not fully understood and their interpretation remains controversial. In this work, we have investigated the dynamics of water permeation through pristine graphene and graphene oxide model membranes that have strong impact on water/alcohol separation. We have carried out fully atomistic classical molecular dynamics simulations of systems composed of multiple layered graphene-based sheets into contact with a pure water reservoir under controlled thermodynamics conditions (e. g., by varying temperature and pressure values). We have systematically analysed how the transport dynamics of the confined nanofluids depend on the interlayer distances and the role of the oxide functional groups. Our results show the water flux is much more effective for graphene than for graphene oxide membranes. These results can be attributed to the H-bonds formation between oxide functional groups and water, which traps the water molecules and precludes ultrafast water transport through the nanochannels.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Cristiano F Woellner Daiane Damasceno Borges, Pedro AS Autreto
Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors Journal Article
In: Carbon, vol. 127, pp. 280-286, 2018.
@article{Borges2018,
title = {Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors},
author = {Daiane Damasceno Borges, Cristiano F Woellner, Pedro AS Autreto, Douglas S Galvao},
url = {https://www.sciencedirect.com/science/article/pii/S000862231731134X},
doi = {https://doi.org/10.1016/j.carbon.2017.11.020},
year = {2018},
date = {2018-02-01},
journal = {Carbon},
volume = {127},
pages = {280-286},
abstract = {xperimental evidence has shown that graphene oxide (GO) can be impermeable to liquids, vapors and gases, while it allows a fast permeation of water molecules. Theoretical studies to understand the filtration mechanisms come mostly from water desalination, while very few works have been dedicated to alcohol dehydration. In this work, we have investigated the molecular level mechanism underlying the alcohol/water separation inside GO membranes. A series of Molecular Dynamics and Grand-Canonical Monte Carlo simulations were carried out to probe the ethanol/water and methanol/water separation through GO membranes composed of multiple layered graphene-based films with different interlayer distance values and number of oxygen-containing functional groups. Our results show that the size exclusion and membrane affinities are not sufficient to explain the selectivity. Besides that, the favorable water molecular arrangement inside GO 2D-channels forming a robust H-bond network and the fast water permeation are crucial for an effective separation mechanism. In other words, the separation phenomenon is not only governed by membrane affinities (enthalpic mechanisms) but mainly by the geometry and size factors (entropic mechanisms). Our findings are consistent with the available experimental data and contribute to clarify important aspects of the separation behavior of confined alcohol/water in GO membranes.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Borges, Daiane Damasceno; Woellner, Cristiano F; Autreto, Pedro AS; Galvao, Douglas S
2017, (preprint arXiv:1702.00250).
@online{Borges2017b,
title = {Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors},
author = {Borges, Daiane Damasceno and Woellner, Cristiano F and Autreto, Pedro AS and Galvao, Douglas S},
url = {https://arxiv.org/abs/1706.06213},
year = {2017},
date = {2017-06-19},
abstract = {Experimental evidences have shown that graphene oxide (GO) can be impermeable to liquids, vapors and gases, while it allows a fast permeation of water molecules. The understanding of filtration mechanisms came mostly from studies dedicated to water desalination, while very few works have been dedicated to distilling alcohols. In this work, we have investigated the molecular level mechanism underlying the alcohol/water separation inside GO membranes. A series of molecular dynamics and Grand-Canonical Monte Carlo simulations were carried out to probe the ethanol/water and methanol/water separation through GO membranes composed of multiple layered graphene-based sheets with different interlayer distance values and number of oxygen-containing functional groups. Our results show that the size exclusion and membrane affinities are not sufficient to explain the selectivity. Besides that, the favorable water molecular arrangement inside GO 2D-channels forming a robust H-bond network and the fast water diffusion are crucial for an effective separation mechanism. In other words, the separation phenomenon is not only governed by affinities with the membrane (enthalpic mechanisms) but mainly by the geometry and size factors (entropic mechanisms). We verified that the 2D geometry channel with optimal interlayer distance are key factors for designing more efficient alcohol-water separation membranes. Our findings are consistent with the available experimental data and contribute to clarify important aspects of the separation behavior of confined alcohol/water in GO membranes.},
note = {preprint arXiv:1702.00250},
keywords = {},
pubstate = {published},
tppubtype = {online}
}
2018
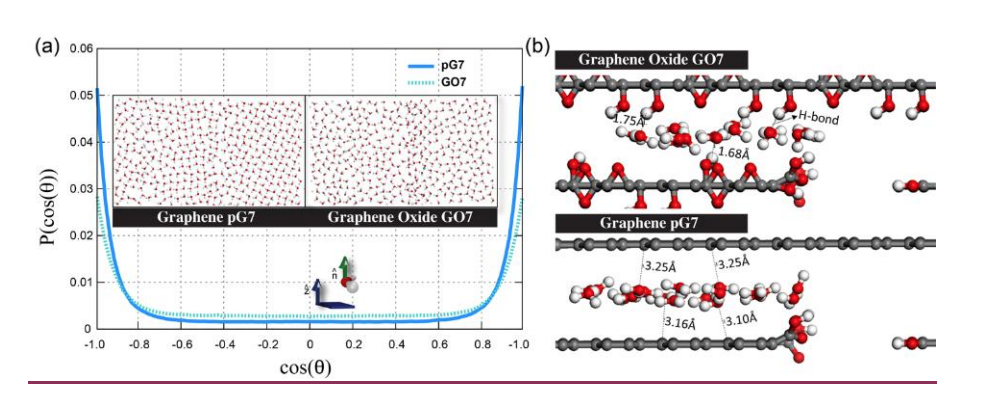
Borges, Daiane Damasceno; Woellner, Cristiano F.; Autreto, Pedro A. S.; Galvao, Douglas S.
Water/alcohol separation via layered oxide graphene membranes Journal Article
In: MRS Advances, vol. 3, no. 1-2, pp. 109-114, 2018.
Abstract | Links | BibTeX | Tags: Filtration, Graphene, Molecular Dynamics
@article{Borges2018d,
title = {Water/alcohol separation via layered oxide graphene membranes},
author = {Daiane Damasceno Borges and Cristiano F. Woellner and Pedro A. S. Autreto and Douglas S. Galvao},
url = {https://www.cambridge.org/core/journals/mrs-advances/article/wateralcohol-separation-in-graphene-oxide-membranes-insights-from-molecular-dynamics-and-monte-carlo-simulations/C61C66FF48D35EB2DB3408ACCE96C41A},
doi = { https://doi.org/10.1557/adv.2018.192},
year = {2018},
date = {2018-02-13},
journal = {MRS Advances},
volume = {3},
number = {1-2},
pages = {109-114},
abstract = {Graphene-based membranes have been investigated as promising candidates for water filtration and gas separation applications. Experimental evidences have shown that graphene oxide can be impermeable to liquids, vapors and gases, while allowing a fast permeation of water molecules. This phenomenon has been attributed to the formation of a network of nano capillaries that allow nearly frictionless water flow while blocking other molecules by steric hindrance effects. It is supposed that water molecules are transported through the percolated two-dimensional channels formed between graphene-based sheets. Although these channels allow fast water permeation in such materials, the flow rates are strongly dependent on how the membranes are fabricated. Also, some fundamental issues regarding the nanoscale mechanisms of water permeation are still not fully understood and their interpretation remains controversial. In this work, we have investigated the dynamics of water permeation through pristine graphene and graphene oxide model membranes that have strong impact on water/alcohol separation. We have carried out fully atomistic classical molecular dynamics simulations of systems composed of multiple layered graphene-based sheets into contact with a pure water reservoir under controlled thermodynamics conditions (e. g., by varying temperature and pressure values). We have systematically analysed how the transport dynamics of the confined nanofluids depend on the interlayer distances and the role of the oxide functional groups. Our results show the water flux is much more effective for graphene than for graphene oxide membranes. These results can be attributed to the H-bonds formation between oxide functional groups and water, which traps the water molecules and precludes ultrafast water transport through the nanochannels.},
keywords = {Filtration, Graphene, Molecular Dynamics},
pubstate = {published},
tppubtype = {article}
}
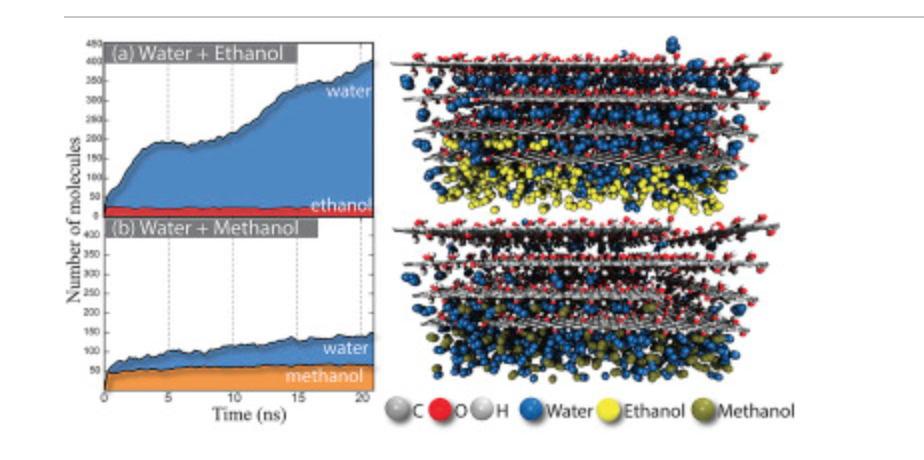
Cristiano F Woellner Daiane Damasceno Borges, Pedro AS Autreto
Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors Journal Article
In: Carbon, vol. 127, pp. 280-286, 2018.
Abstract | Links | BibTeX | Tags: Filtration, Graphene, Molecular Dynamics
@article{Borges2018,
title = {Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors},
author = {Daiane Damasceno Borges, Cristiano F Woellner, Pedro AS Autreto, Douglas S Galvao},
url = {https://www.sciencedirect.com/science/article/pii/S000862231731134X},
doi = {https://doi.org/10.1016/j.carbon.2017.11.020},
year = {2018},
date = {2018-02-01},
journal = {Carbon},
volume = {127},
pages = {280-286},
abstract = {xperimental evidence has shown that graphene oxide (GO) can be impermeable to liquids, vapors and gases, while it allows a fast permeation of water molecules. Theoretical studies to understand the filtration mechanisms come mostly from water desalination, while very few works have been dedicated to alcohol dehydration. In this work, we have investigated the molecular level mechanism underlying the alcohol/water separation inside GO membranes. A series of Molecular Dynamics and Grand-Canonical Monte Carlo simulations were carried out to probe the ethanol/water and methanol/water separation through GO membranes composed of multiple layered graphene-based films with different interlayer distance values and number of oxygen-containing functional groups. Our results show that the size exclusion and membrane affinities are not sufficient to explain the selectivity. Besides that, the favorable water molecular arrangement inside GO 2D-channels forming a robust H-bond network and the fast water permeation are crucial for an effective separation mechanism. In other words, the separation phenomenon is not only governed by membrane affinities (enthalpic mechanisms) but mainly by the geometry and size factors (entropic mechanisms). Our findings are consistent with the available experimental data and contribute to clarify important aspects of the separation behavior of confined alcohol/water in GO membranes.},
keywords = {Filtration, Graphene, Molecular Dynamics},
pubstate = {published},
tppubtype = {article}
}
2017
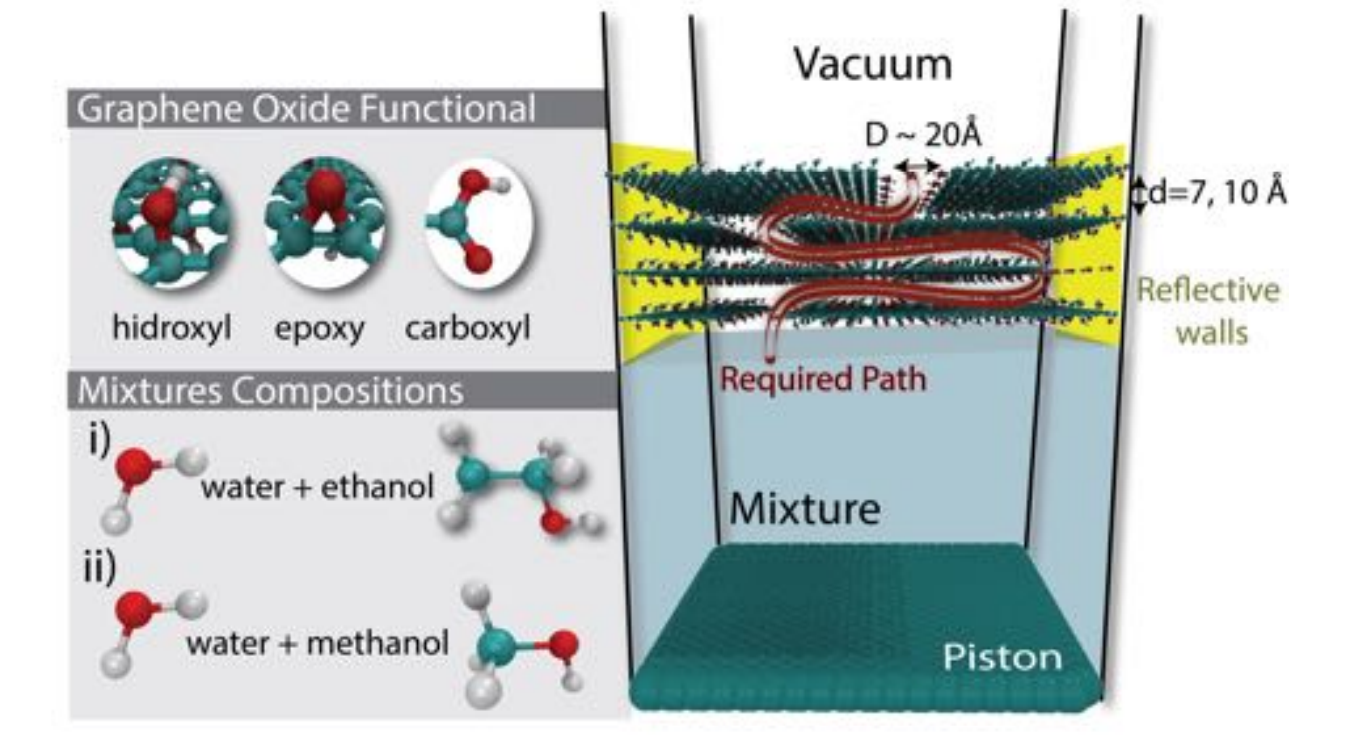
Borges, Daiane Damasceno; Woellner, Cristiano F; Autreto, Pedro AS; Galvao, Douglas S
2017, (preprint arXiv:1702.00250).
Abstract | Links | BibTeX | Tags: Filtration, Graphene Membranes, Molecular Dyanmics
@online{Borges2017b,
title = {Insights on the mechanism of water-alcohol separation in multilayer graphene oxide membranes: entropic versus enthalpic factors},
author = {Borges, Daiane Damasceno and Woellner, Cristiano F and Autreto, Pedro AS and Galvao, Douglas S},
url = {https://arxiv.org/abs/1706.06213},
year = {2017},
date = {2017-06-19},
abstract = {Experimental evidences have shown that graphene oxide (GO) can be impermeable to liquids, vapors and gases, while it allows a fast permeation of water molecules. The understanding of filtration mechanisms came mostly from studies dedicated to water desalination, while very few works have been dedicated to distilling alcohols. In this work, we have investigated the molecular level mechanism underlying the alcohol/water separation inside GO membranes. A series of molecular dynamics and Grand-Canonical Monte Carlo simulations were carried out to probe the ethanol/water and methanol/water separation through GO membranes composed of multiple layered graphene-based sheets with different interlayer distance values and number of oxygen-containing functional groups. Our results show that the size exclusion and membrane affinities are not sufficient to explain the selectivity. Besides that, the favorable water molecular arrangement inside GO 2D-channels forming a robust H-bond network and the fast water diffusion are crucial for an effective separation mechanism. In other words, the separation phenomenon is not only governed by affinities with the membrane (enthalpic mechanisms) but mainly by the geometry and size factors (entropic mechanisms). We verified that the 2D geometry channel with optimal interlayer distance are key factors for designing more efficient alcohol-water separation membranes. Our findings are consistent with the available experimental data and contribute to clarify important aspects of the separation behavior of confined alcohol/water in GO membranes.},
note = {preprint arXiv:1702.00250},
keywords = {Filtration, Graphene Membranes, Molecular Dyanmics},
pubstate = {published},
tppubtype = {online}
}
http://scholar.google.com/citations?hl=en&user=95SvbM8AAAAJ


